Iron in PDB 8r8x: Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay
Protein crystallography data
The structure of Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay, PDB code: 8r8x
was solved by
T.Barends,
I.Schlichting,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 9.95 / 1.40 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 63, 28.4, 35.3, 90, 107, 90 |
| R / Rfree (%) | 19.4 / 21.8 |
Iron Binding Sites:
The binding sites of Iron atom in the Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay
(pdb code 8r8x). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay, PDB code: 8r8x:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay, PDB code: 8r8x:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 8r8x
Go back to
Iron binding site 1 out
of 2 in the Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay
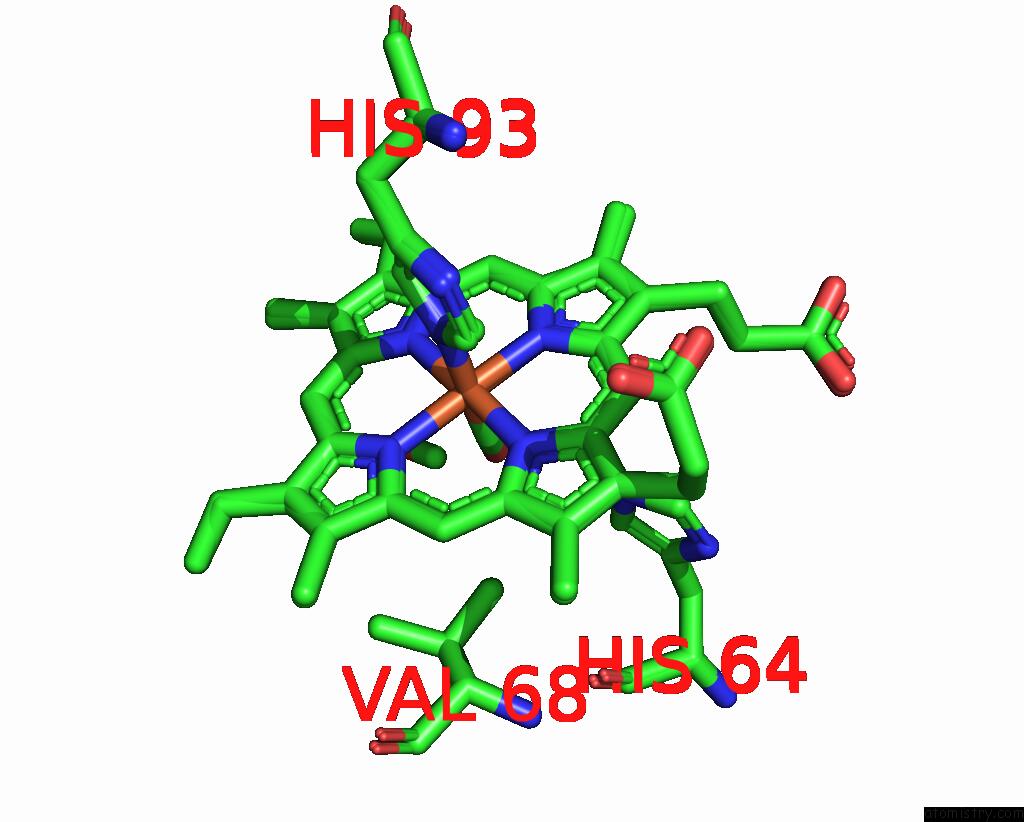
Mono view
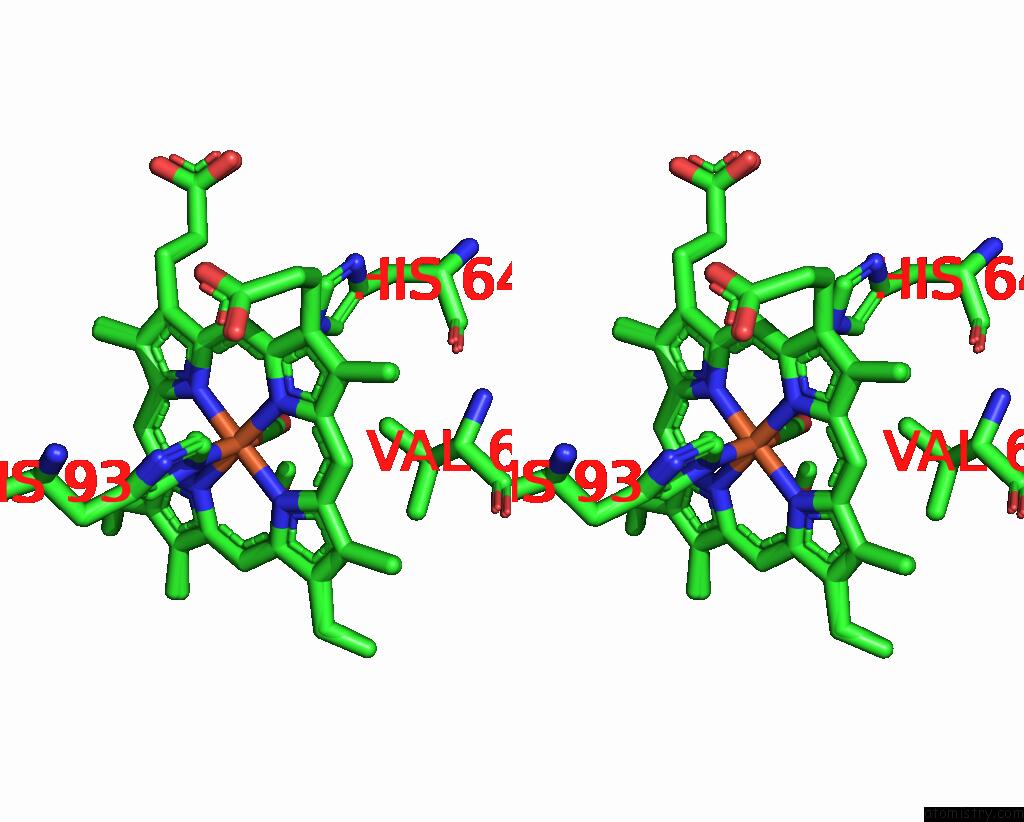
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay within 5.0Å range:
|
Iron binding site 2 out of 2 in 8r8x
Go back to
Iron binding site 2 out
of 2 in the Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay
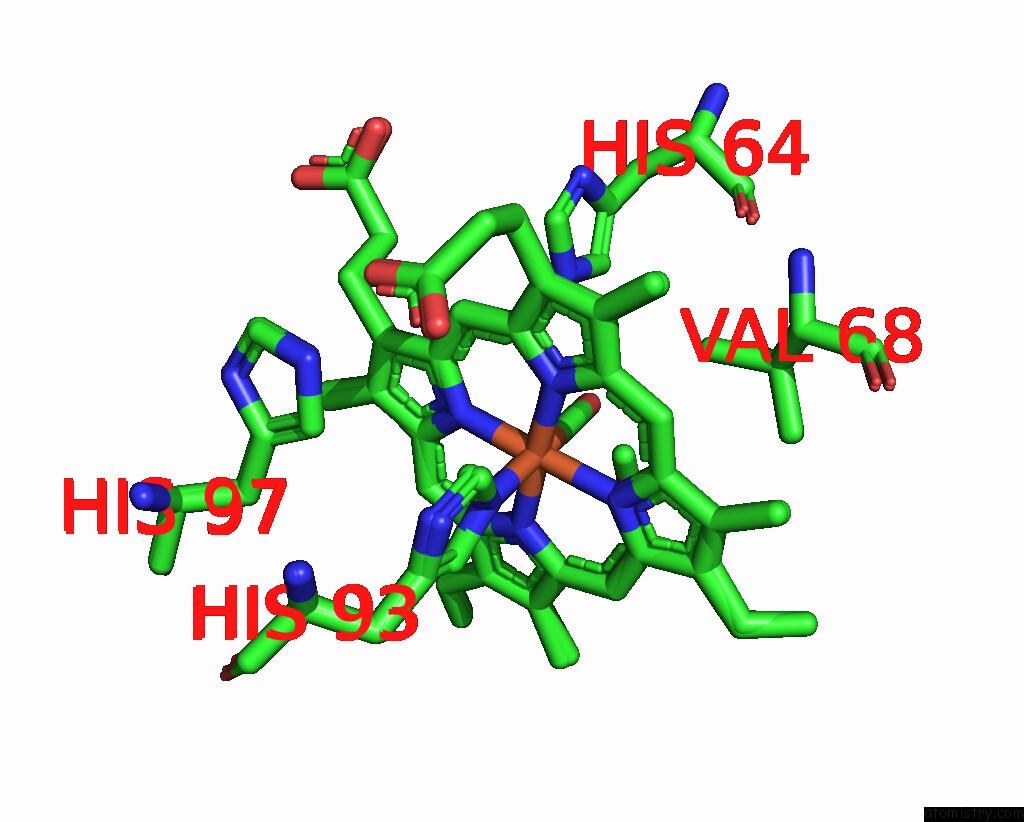
Mono view
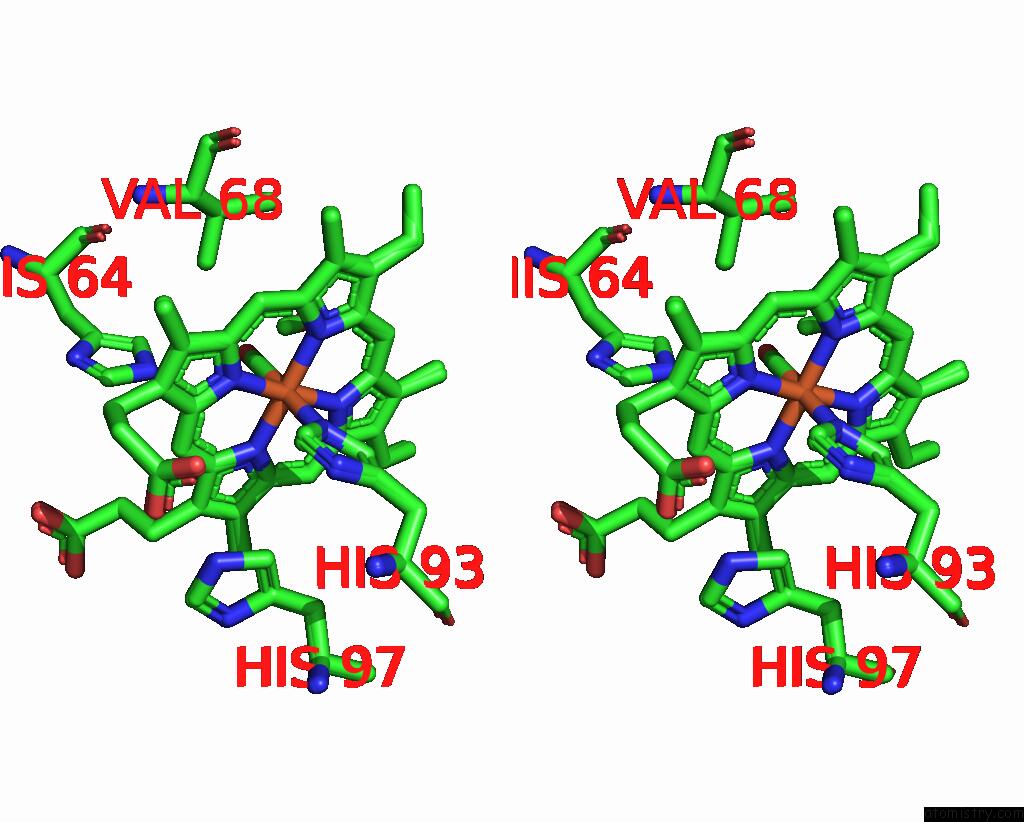
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Multicopy-Refined Carboxymyoglobin Photolysis Time Series at 5 Mj/CM2 Laser Fluence, 327 Fs Time Delay within 5.0Å range:
|
Reference:
T.R.M.Barends,
A.Gorel,
S.Bhattacharyya,
G.Schiro,
C.Bacellar,
C.Cirelli,
J.P.Colletier,
L.Foucar,
M.L.Grunbein,
E.Hartmann,
M.Hilpert,
J.M.Holton,
P.J.M.Johnson,
M.Kloos,
G.Knopp,
B.Marekha,
K.Nass,
G.Nass Kovacs,
D.Ozerov,
M.Stricker,
M.Weik,
R.B.Doak,
R.L.Shoeman,
C.J.Milne,
M.Huix-Rotllant,
M.Cammarata,
I.Schlichting.
Influence of Pump Laser Fluence on Ultrafast Myoglobin Structural Dynamics. Nature V. 626 905 2024.
ISSN: ESSN 1476-4687
PubMed: 38355794
DOI: 10.1038/S41586-024-07032-9
Page generated: Sat Aug 10 16:19:19 2024
ISSN: ESSN 1476-4687
PubMed: 38355794
DOI: 10.1038/S41586-024-07032-9
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1