Iron »
PDB 8wgh-8xcg »
8wzh »
Iron in PDB 8wzh: Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
Enzymatic activity of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
All present enzymatic activity of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation:
1.11.1.6;
1.11.1.6;
Iron Binding Sites:
The binding sites of Iron atom in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
(pdb code 8wzh). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation, PDB code: 8wzh:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation, PDB code: 8wzh:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 8wzh
Go back to
Iron binding site 1 out
of 4 in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
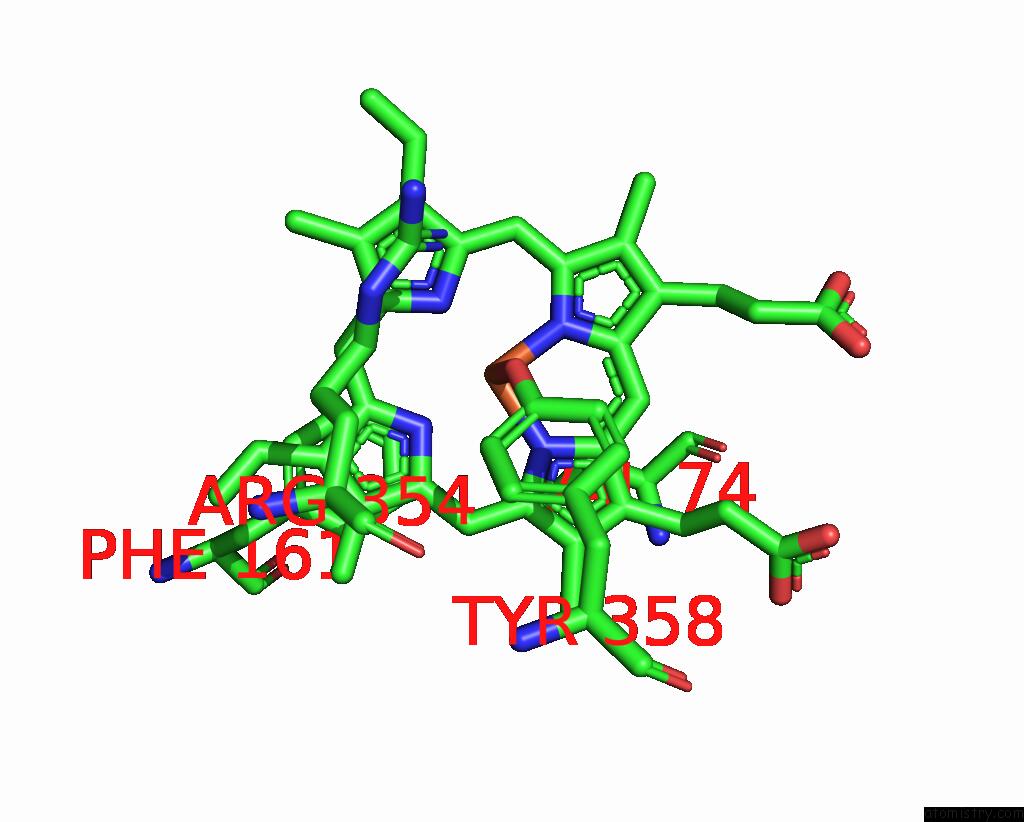
Mono view
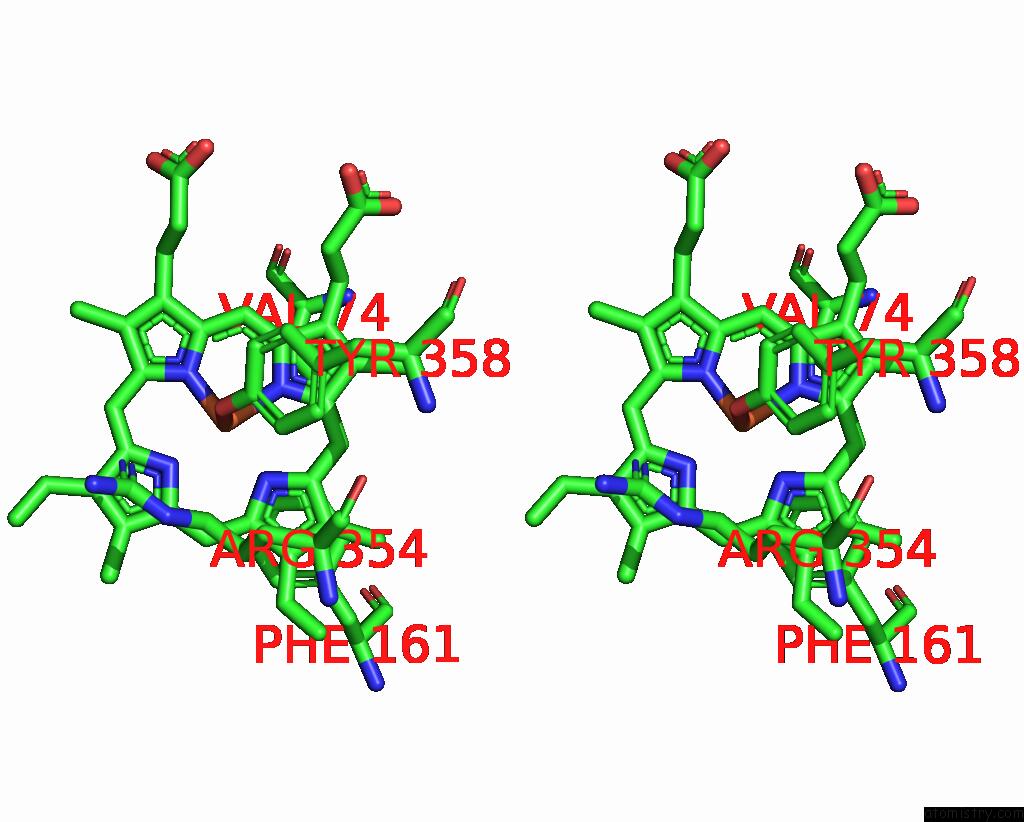
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation within 5.0Å range:
|
Iron binding site 2 out of 4 in 8wzh
Go back to
Iron binding site 2 out
of 4 in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
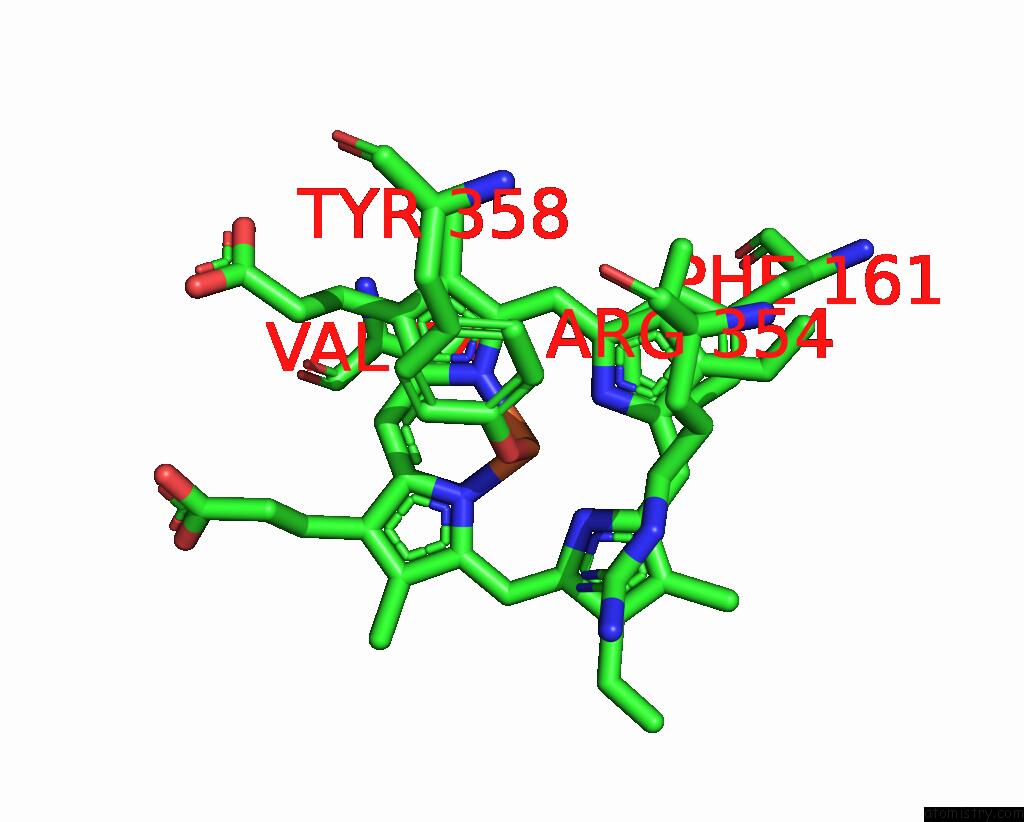
Mono view
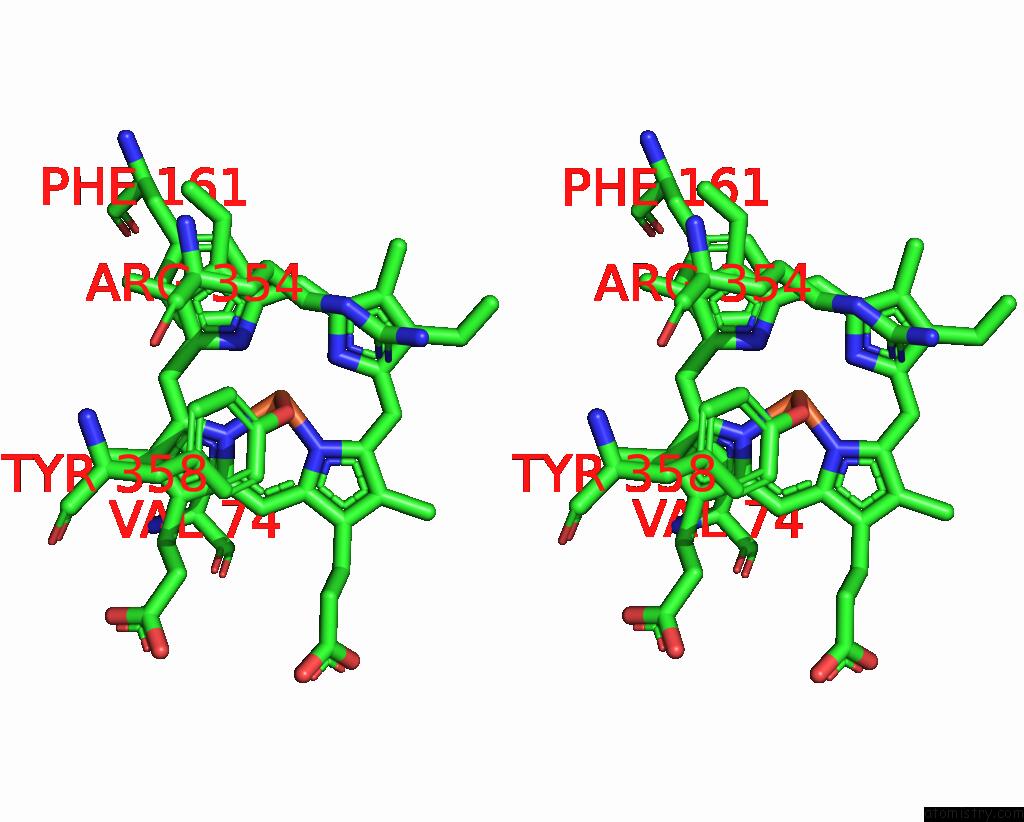
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation within 5.0Å range:
|
Iron binding site 3 out of 4 in 8wzh
Go back to
Iron binding site 3 out
of 4 in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
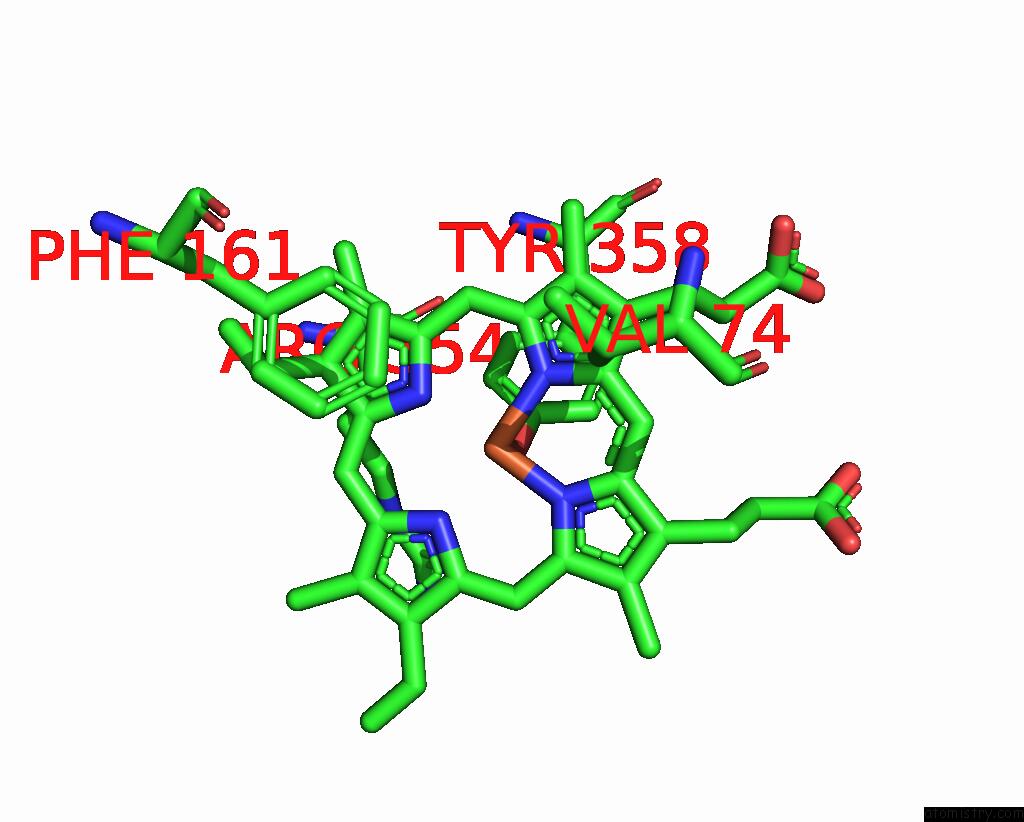
Mono view
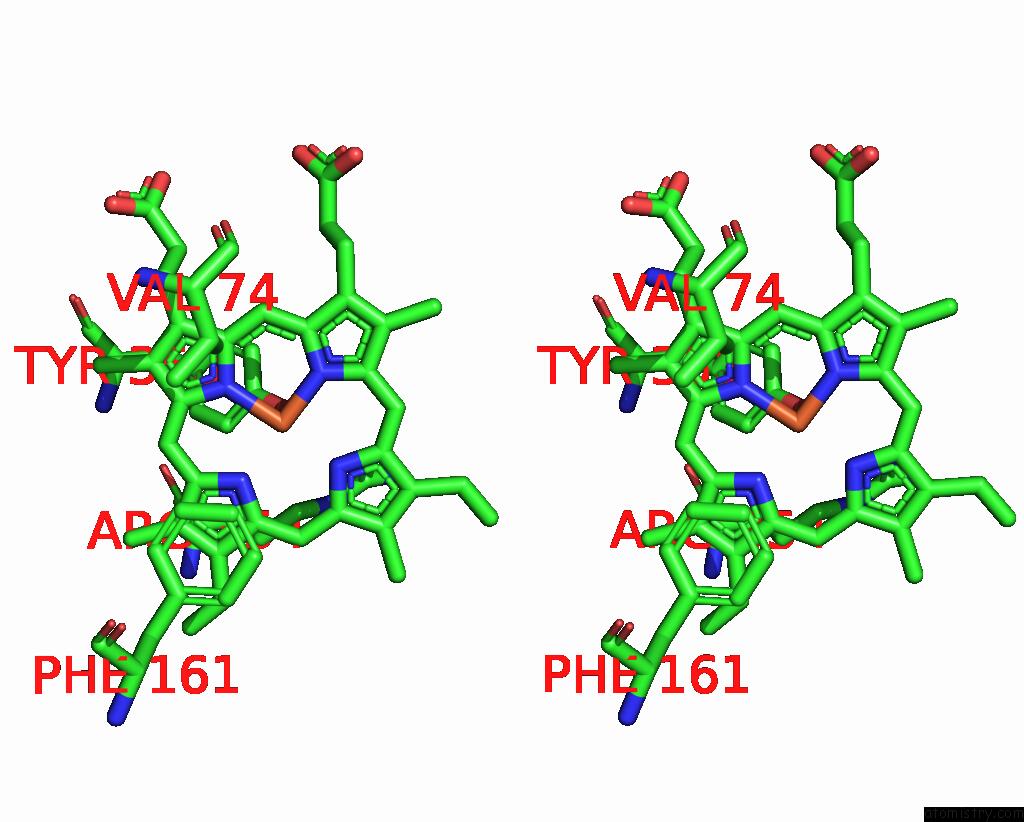
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation within 5.0Å range:
|
Iron binding site 4 out of 4 in 8wzh
Go back to
Iron binding site 4 out
of 4 in the Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation
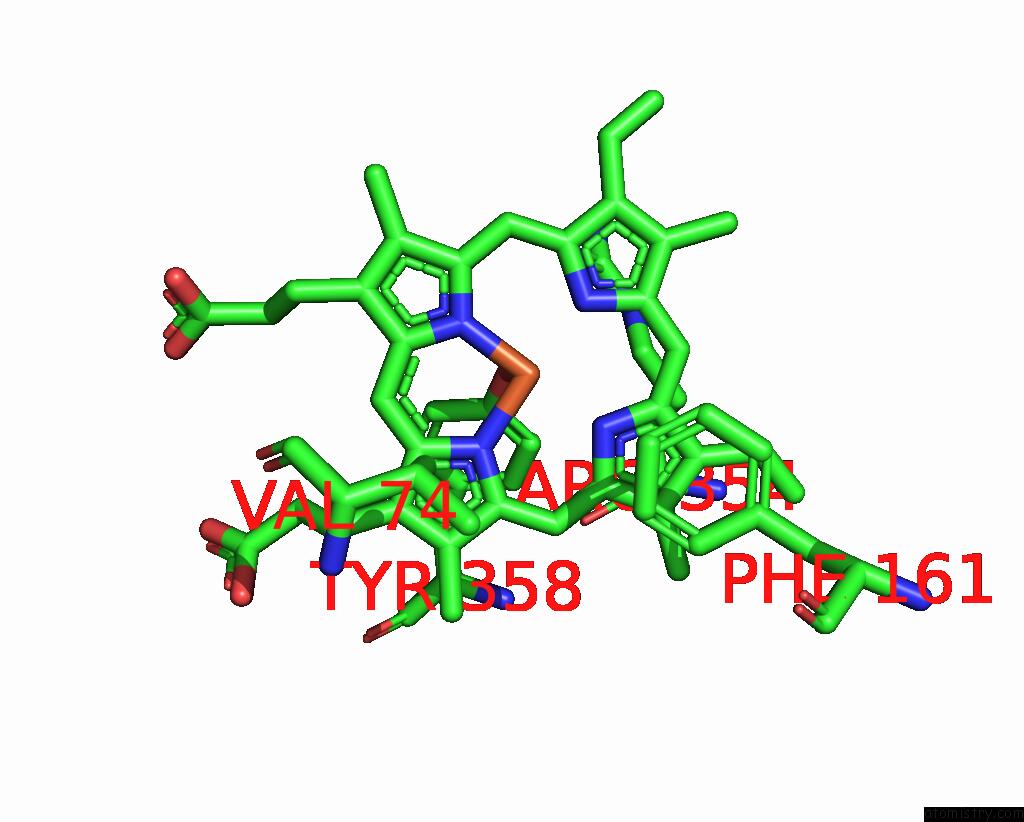
Mono view
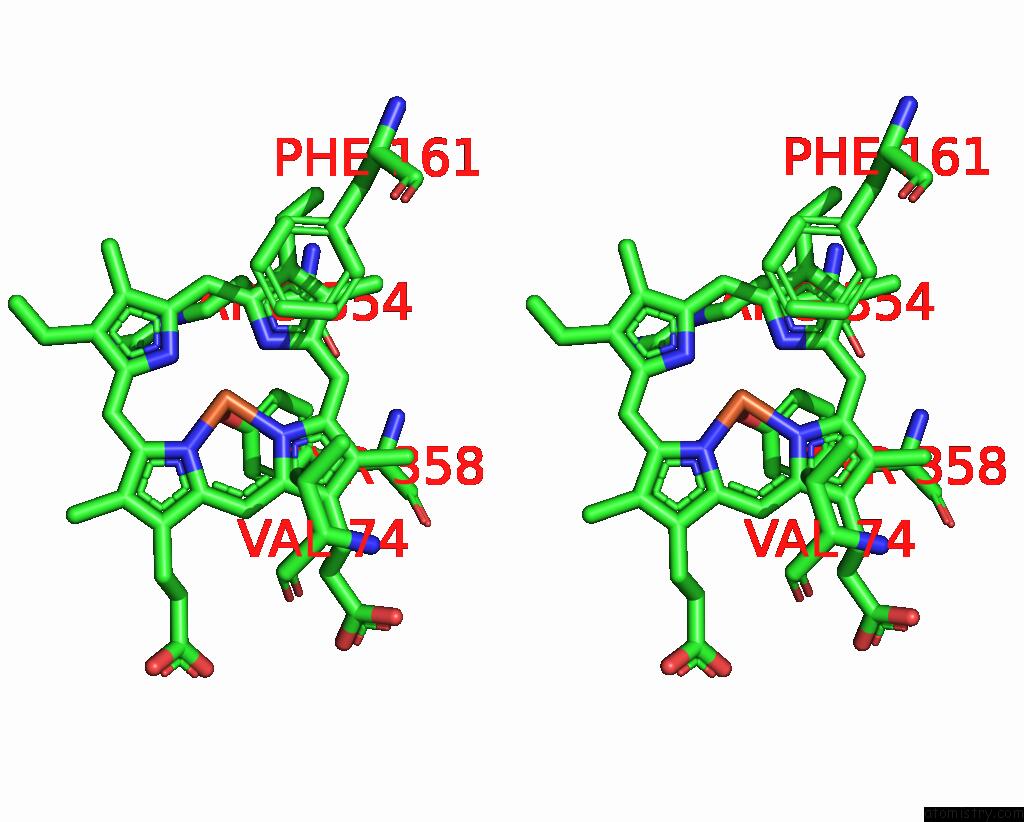
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Human Erythrocyte Catalase with Sls As Additive During Cryo-Em Grid Preparation within 5.0Å range:
|
Reference:
S.Yadav,
K.R.Vinothkumar.
Factors Affecting Macromolecule Orientations in Thin Films Formed in Cryo-Em. Acta Crystallogr D Struct 2024BIOL.
ISSN: ISSN 2059-7983
PubMed: 38935342
DOI: 10.1107/S2059798324005229
Page generated: Fri Aug 8 00:43:52 2025
ISSN: ISSN 2059-7983
PubMed: 38935342
DOI: 10.1107/S2059798324005229
Last articles
Fe in 9CQWFe in 9CQV
Fe in 9CQU
Fe in 9CQT
Fe in 9CQS
Fe in 9CJF
Fe in 9CQR
Fe in 9CQQ
Fe in 9CQP
Fe in 9CQO