Iron in PDB 8xcn: Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
Iron Binding Sites:
The binding sites of Iron atom in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
(pdb code 8xcn). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 6 binding sites of Iron where determined in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A, PDB code: 8xcn:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Iron where determined in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A, PDB code: 8xcn:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6;
Iron binding site 1 out of 6 in 8xcn
Go back to
Iron binding site 1 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
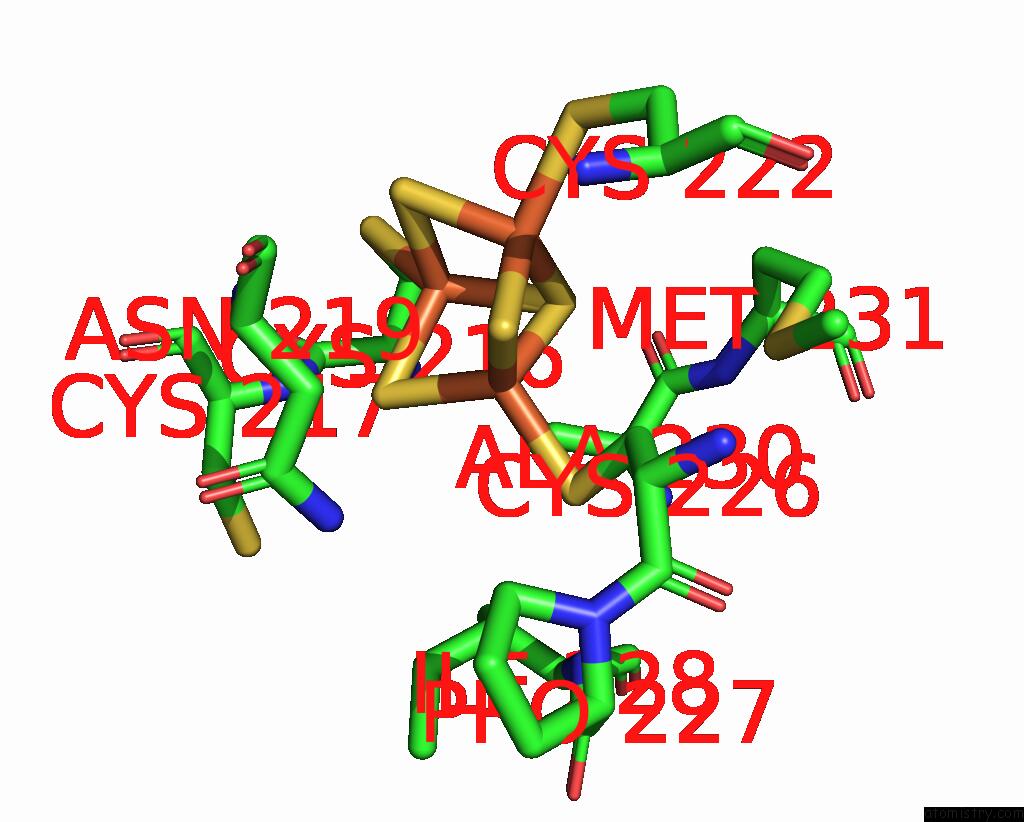
Mono view
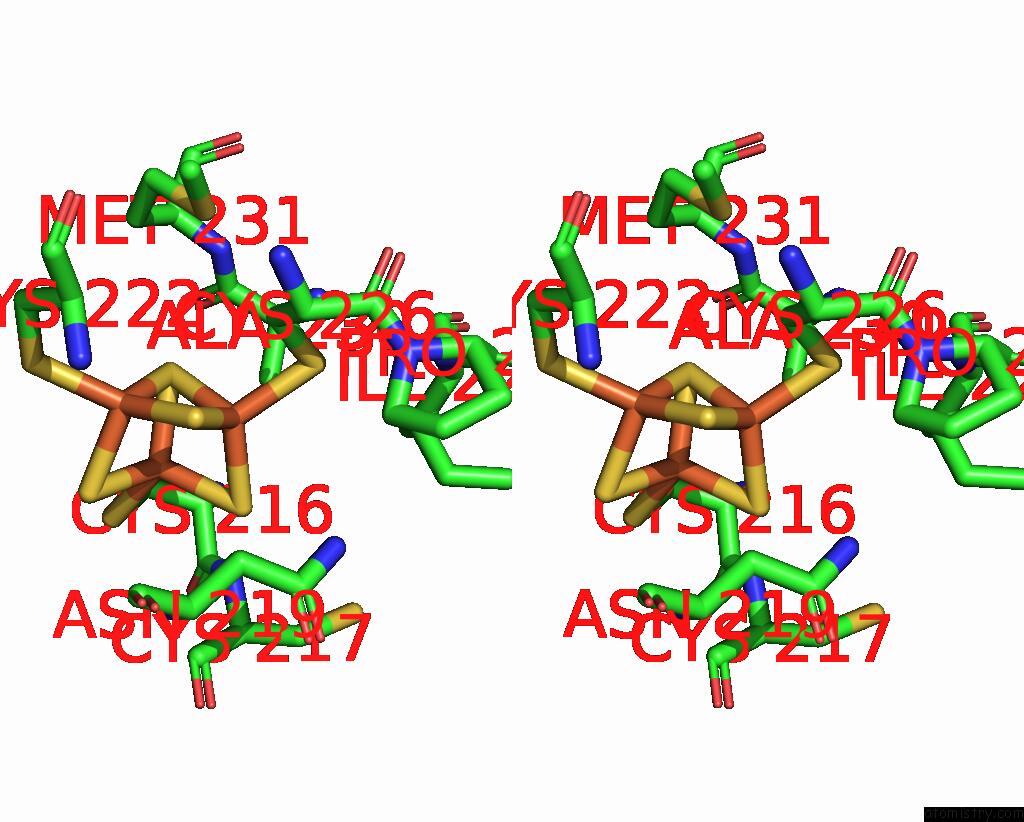
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Iron binding site 2 out of 6 in 8xcn
Go back to
Iron binding site 2 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
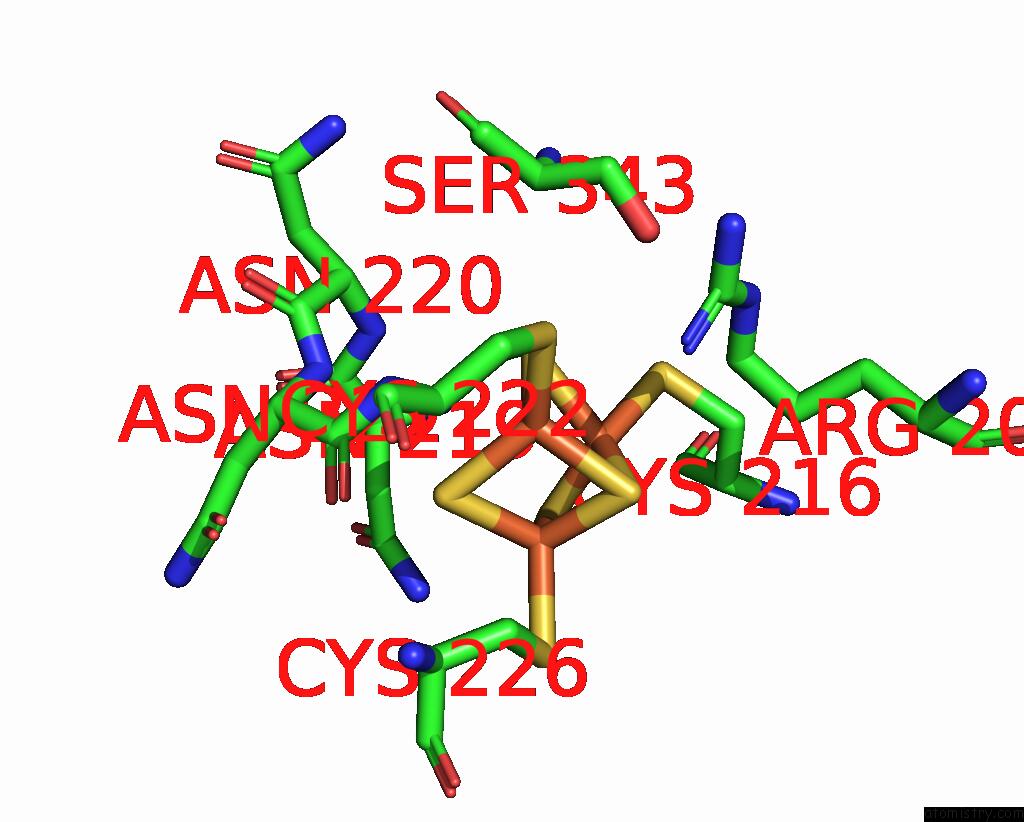
Mono view
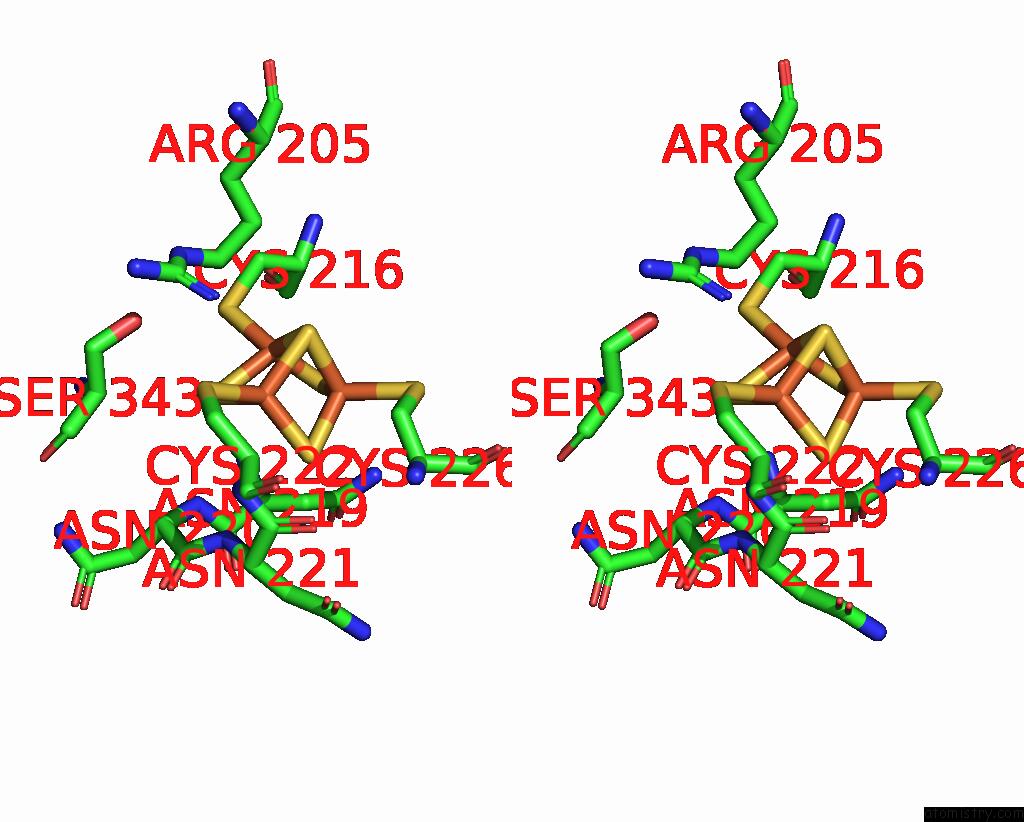
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Iron binding site 3 out of 6 in 8xcn
Go back to
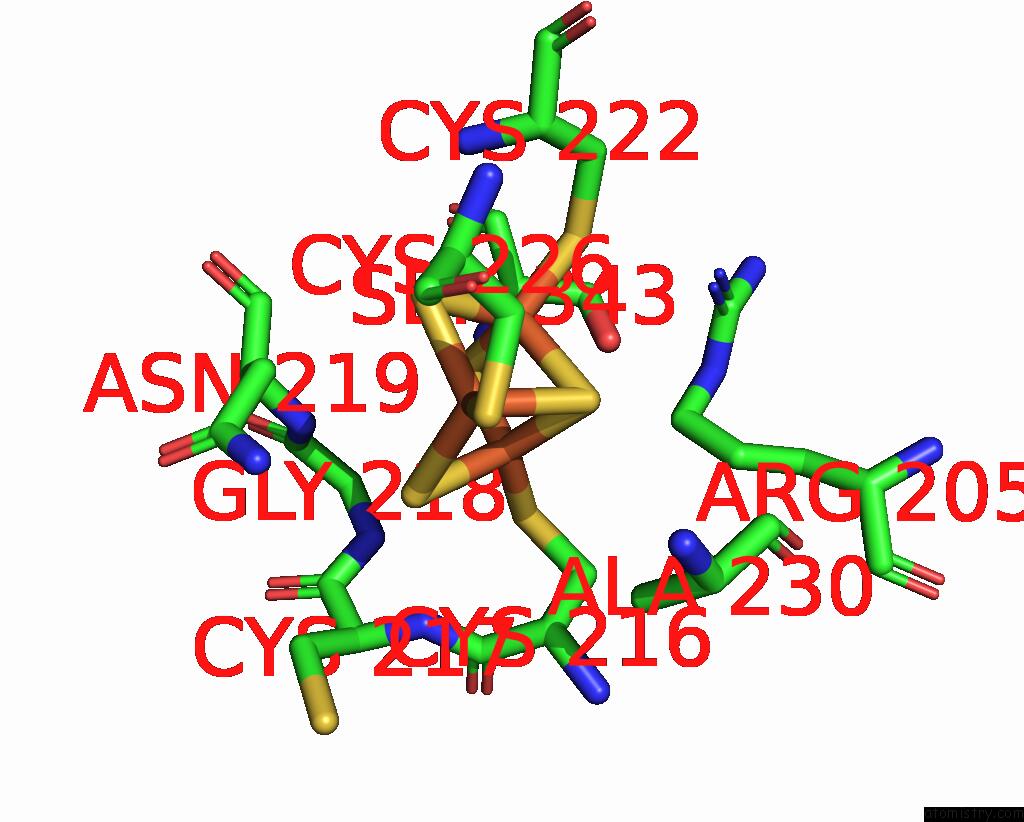
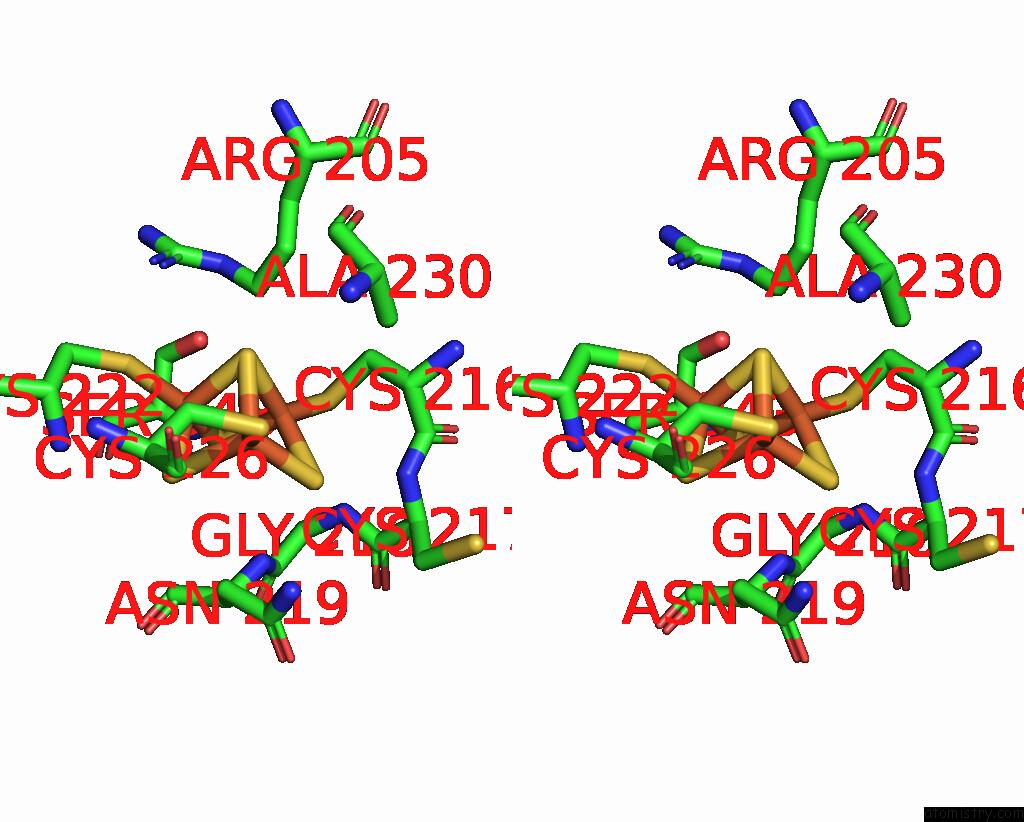
Iron binding site 3 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Iron binding site 4 out of 6 in 8xcn
Go back to
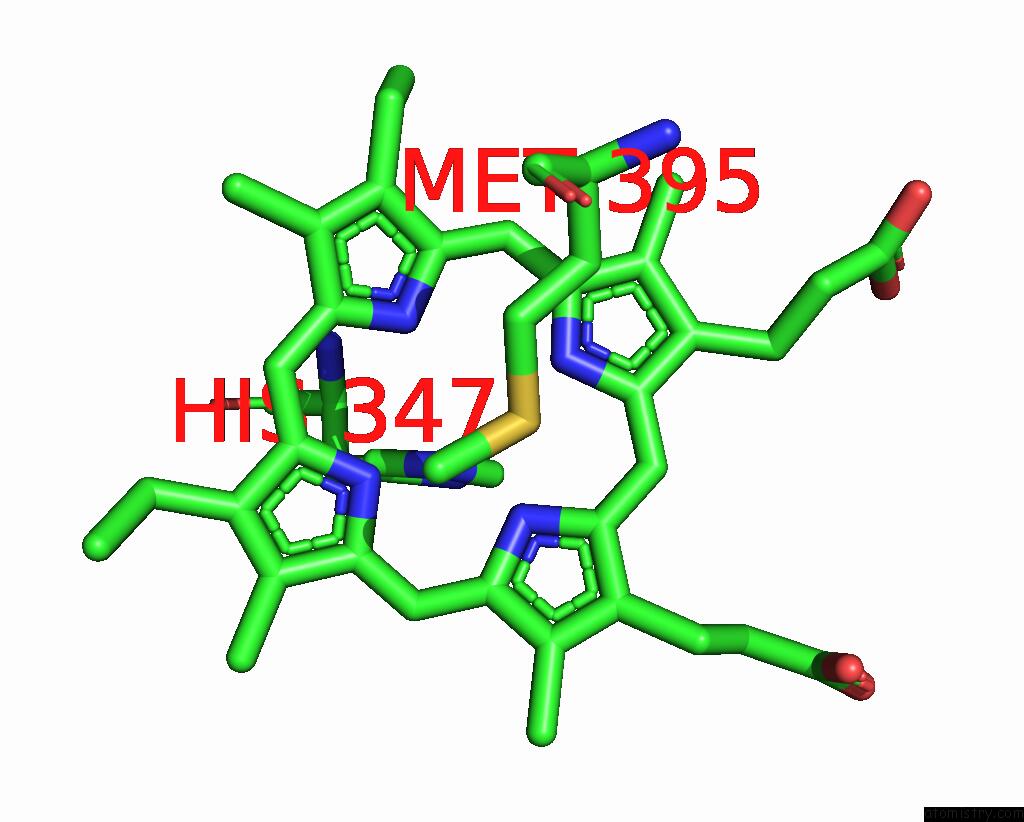
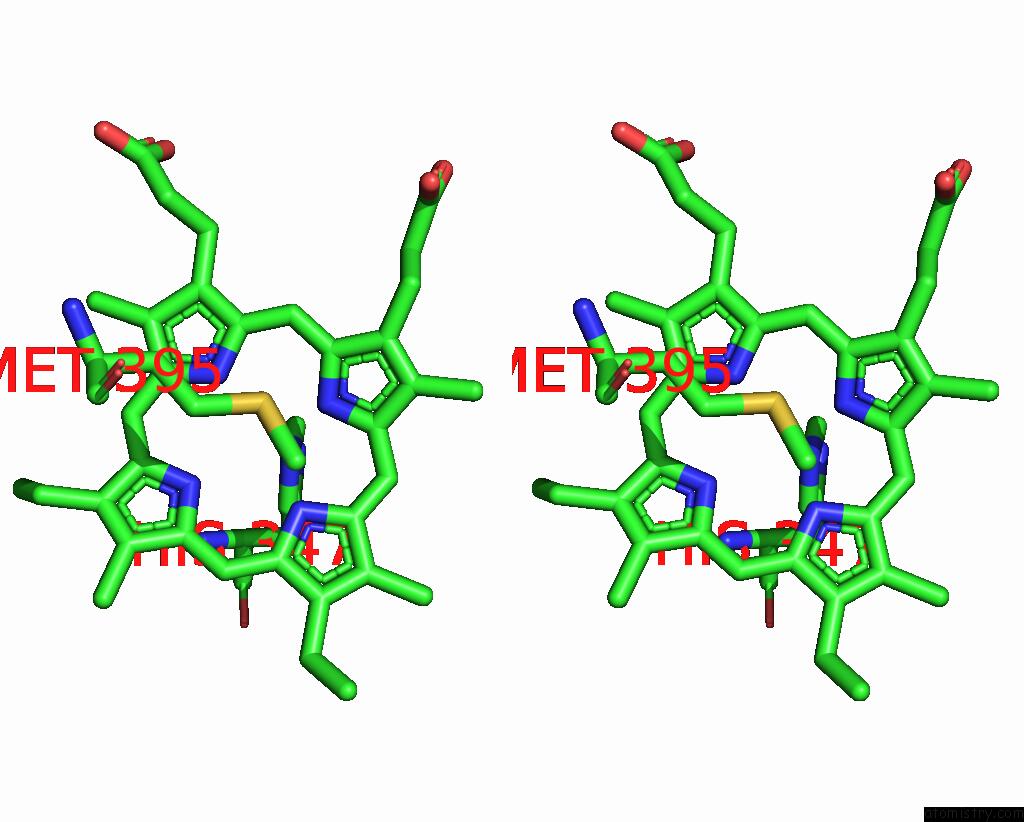
Iron binding site 4 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Iron binding site 5 out of 6 in 8xcn
Go back to
Iron binding site 5 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
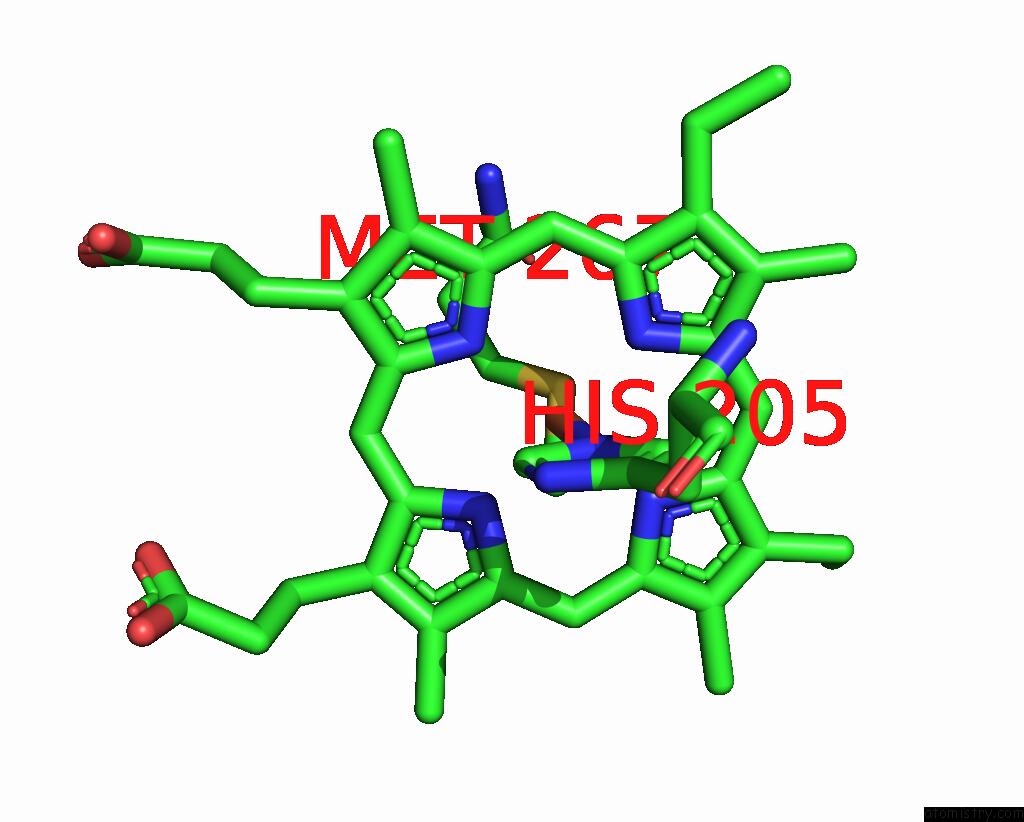
Mono view
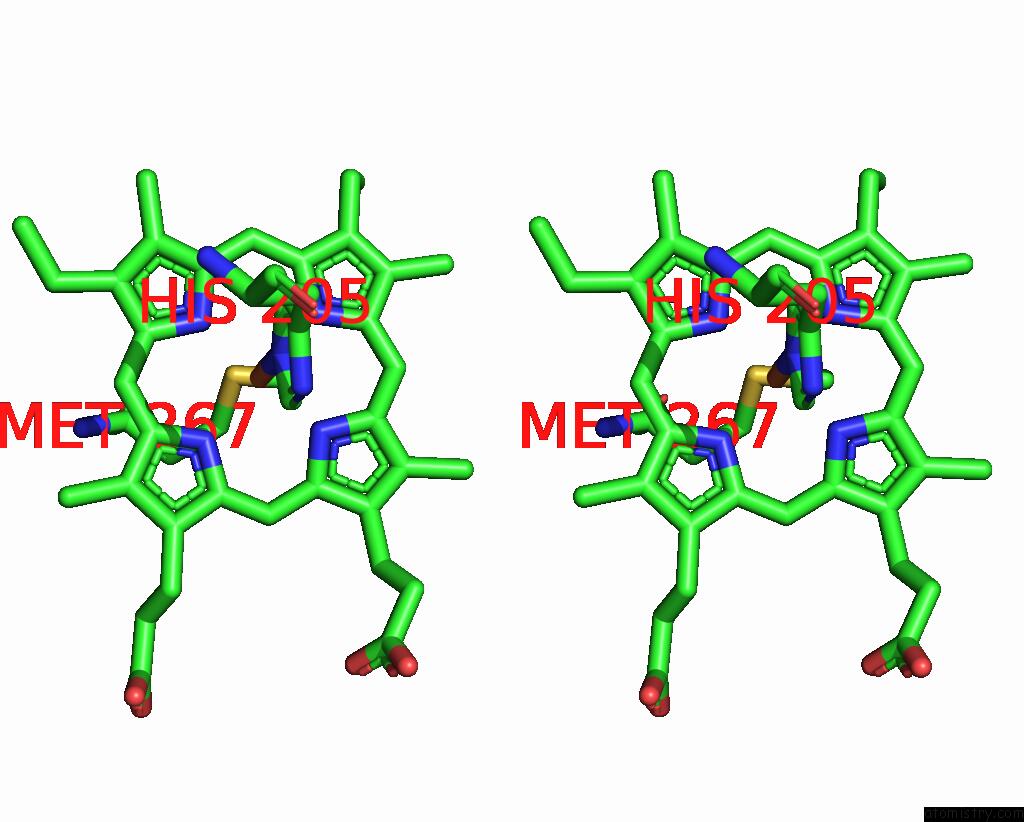
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Iron binding site 6 out of 6 in 8xcn
Go back to
Iron binding site 6 out
of 6 in the Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A
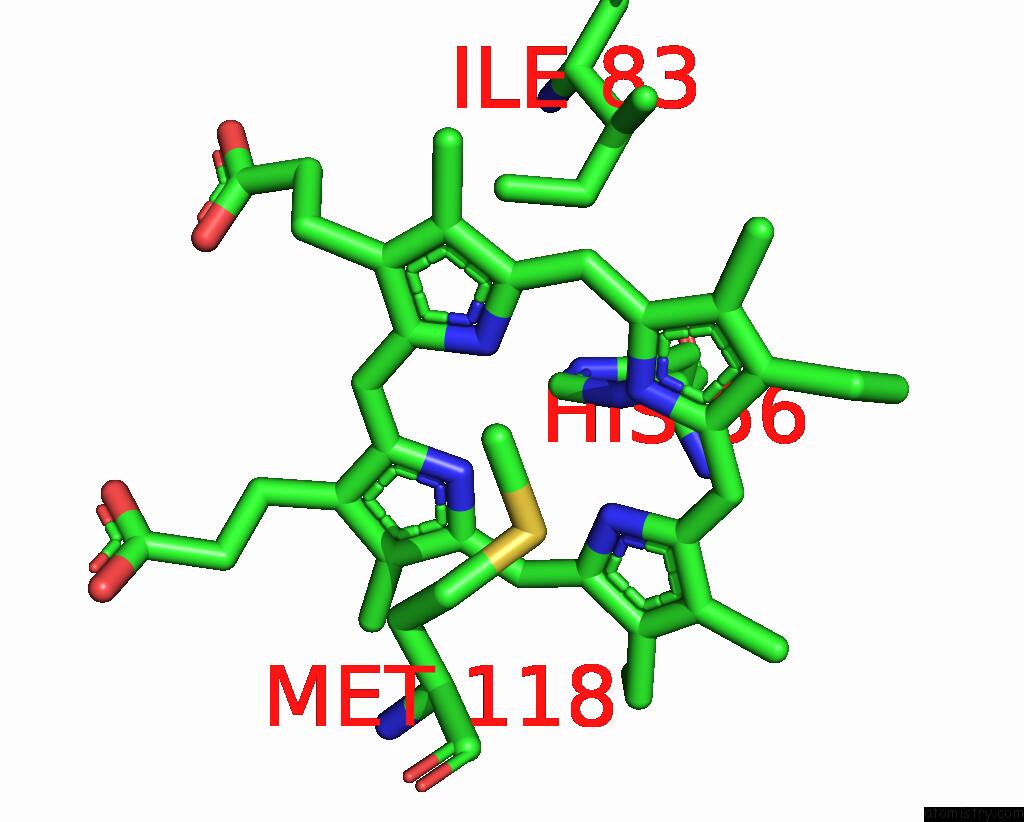
Mono view
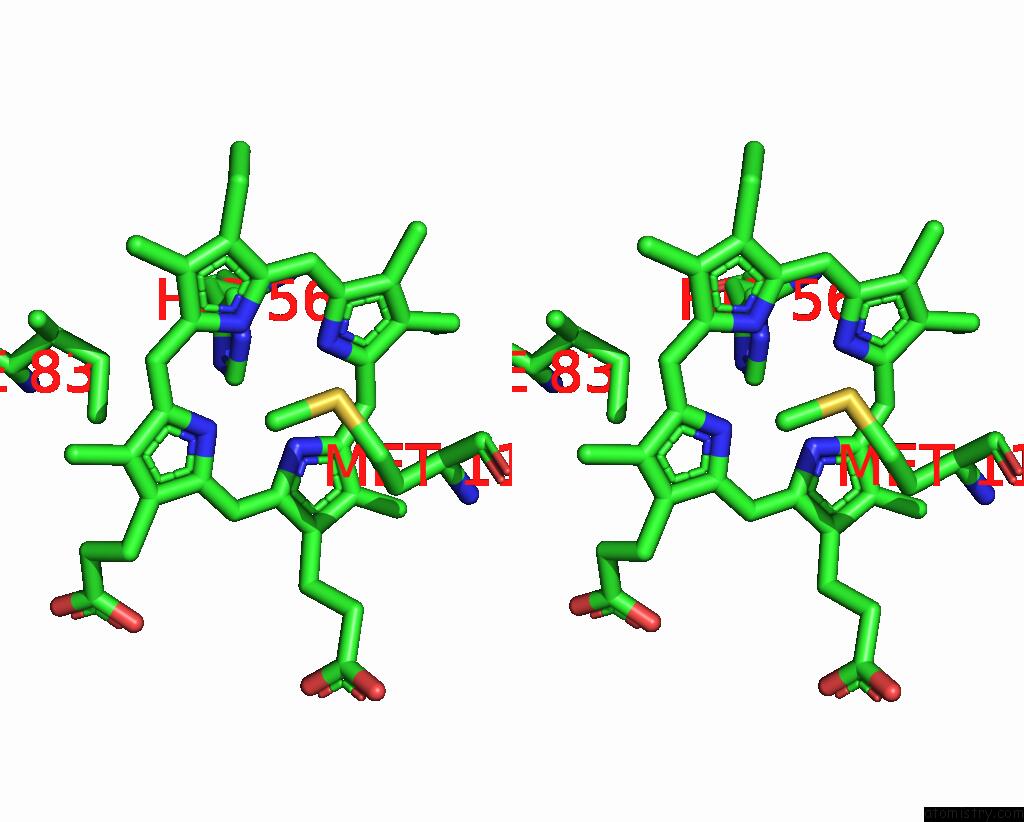
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Cryo-Em Structure of Membrane-Bound Fructose Dehydrogenase From Gluconobacter Japonicus Variant-N1190A within 5.0Å range:
|
Reference:
E.Fukawa,
Y.Suzuki,
T.Adachi,
T.Miyata,
F.Makino,
H.Tanaka,
K.Namba,
K.Sowa,
Y.Kitazumi,
O.Shirai.
Structural and Electrochemical Elucidation of Biocatalytic Mechanisms in Direct Electron Transfer-Type D-Fructose Dehydrogenase. Electrochim Acta V. 490 2024.
DOI: 10.1016/J.ELECTACTA.2024.144271
Page generated: Sat Aug 10 20:27:24 2024
DOI: 10.1016/J.ELECTACTA.2024.144271
Last articles
Zn in 9IRQZn in 9IYX
Zn in 9J8P
Zn in 9IUU
Zn in 9GBF
Zn in 9G2V
Zn in 9G2L
Zn in 9G2X
Zn in 9G2Z
Zn in 9G2K