Iron »
PDB 1fz2-1gek »
1fz6 »
Iron in PDB 1fz6: Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
Enzymatic activity of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
All present enzymatic activity of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol:
1.14.13.25;
1.14.13.25;
Protein crystallography data
The structure of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol, PDB code: 1fz6
was solved by
D.A.Whittington,
M.H.Sazinsky,
S.J.Lippard,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.90 / 2.05 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.670, 171.290, 221.340, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.8 / 24.4 |
Other elements in 1fz6:
The structure of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol also contains other interesting chemical elements:
| Calcium | (Ca) | 3 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
(pdb code 1fz6). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol, PDB code: 1fz6:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol, PDB code: 1fz6:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 1fz6
Go back to
Iron binding site 1 out
of 4 in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
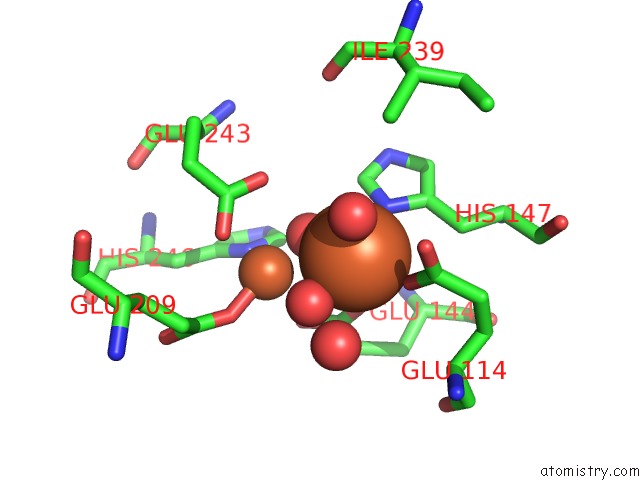
Mono view
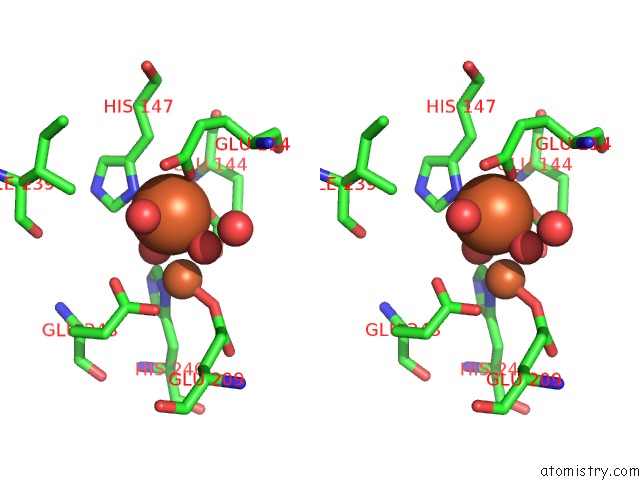
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol within 5.0Å range:
|
Iron binding site 2 out of 4 in 1fz6
Go back to
Iron binding site 2 out
of 4 in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
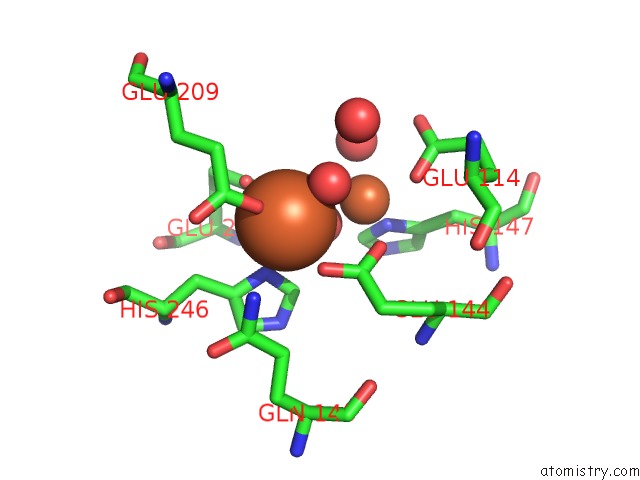
Mono view
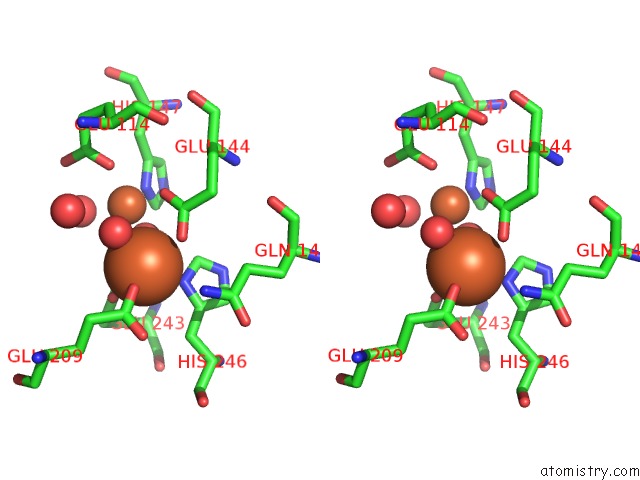
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol within 5.0Å range:
|
Iron binding site 3 out of 4 in 1fz6
Go back to
Iron binding site 3 out
of 4 in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
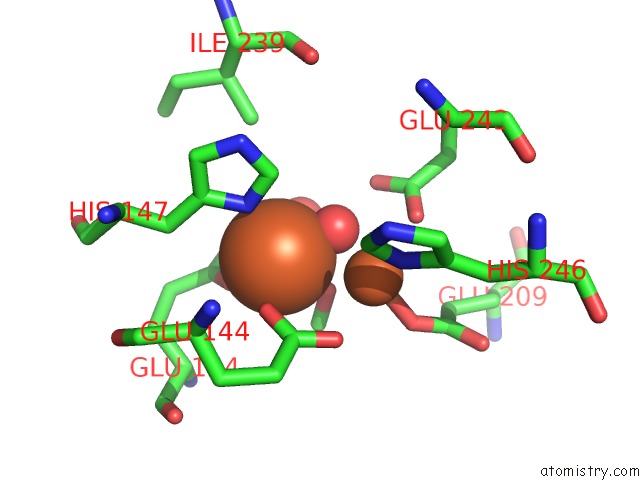
Mono view
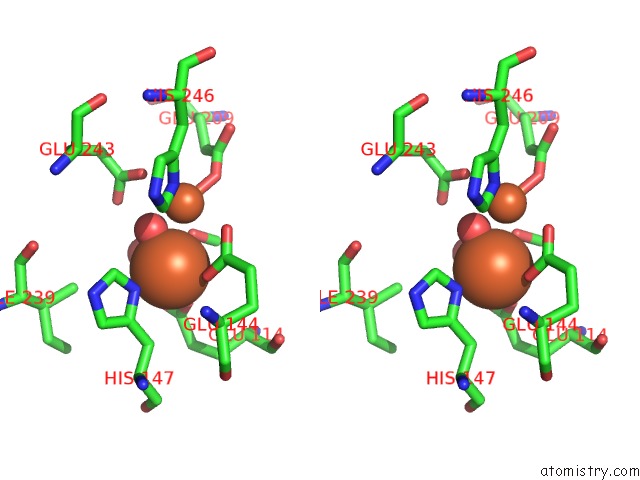
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol within 5.0Å range:
|
Iron binding site 4 out of 4 in 1fz6
Go back to
Iron binding site 4 out
of 4 in the Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol
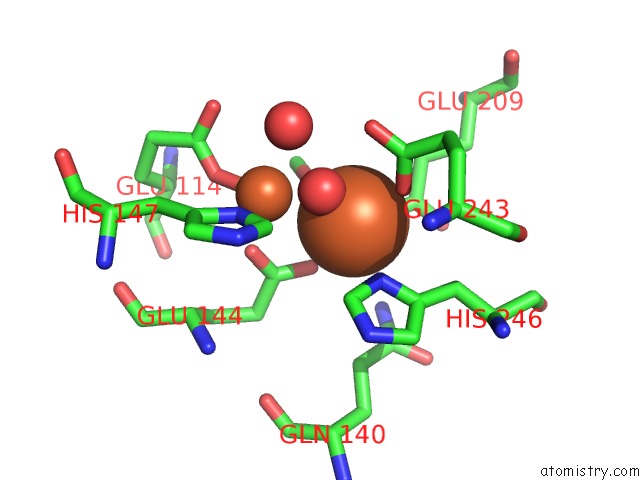
Mono view
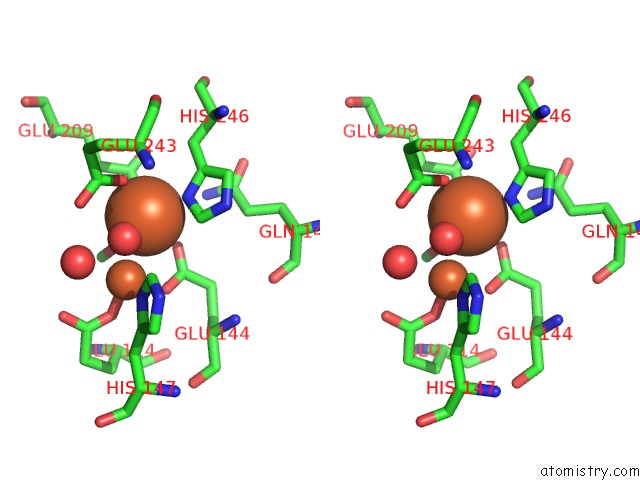
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Methane Monooxygenase Hydroxylase, Form II Soaked in 1 M Methanol within 5.0Å range:
|
Reference:
D.A.Whittington,
M.H.Sazinsky,
S.J.Lippard.
X-Ray Crystal Structure of Alcohol Products Bound at the Active Site of Soluble Methane Monooxygenase Hydroxylase. J.Am.Chem.Soc. V. 123 1794 2001.
ISSN: ISSN 0002-7863
PubMed: 11456795
DOI: 10.1021/JA0031725
Page generated: Wed Jul 16 14:37:19 2025
ISSN: ISSN 0002-7863
PubMed: 11456795
DOI: 10.1021/JA0031725
Last articles
Mg in 7AFNMg in 7AFK
Mg in 7AFH
Mg in 7AF5
Mg in 7AFD
Mg in 7AF8
Mg in 7AF3
Mg in 7AFA
Mg in 7AE3
Mg in 7AE1