Iron »
PDB 1q5e-1qom »
1qks »
Iron in PDB 1qks: Cytochrome CD1 Nitrite Reductase, Oxidised Form
Protein crystallography data
The structure of Cytochrome CD1 Nitrite Reductase, Oxidised Form, PDB code: 1qks
was solved by
V.Fulop,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 1.28 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 106.400, 60.600, 100.200, 90.00, 112.30, 90.00 |
| R / Rfree (%) | 18.5 / 20 |
Iron Binding Sites:
The binding sites of Iron atom in the Cytochrome CD1 Nitrite Reductase, Oxidised Form
(pdb code 1qks). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Cytochrome CD1 Nitrite Reductase, Oxidised Form, PDB code: 1qks:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Cytochrome CD1 Nitrite Reductase, Oxidised Form, PDB code: 1qks:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 1qks
Go back to
Iron binding site 1 out
of 4 in the Cytochrome CD1 Nitrite Reductase, Oxidised Form
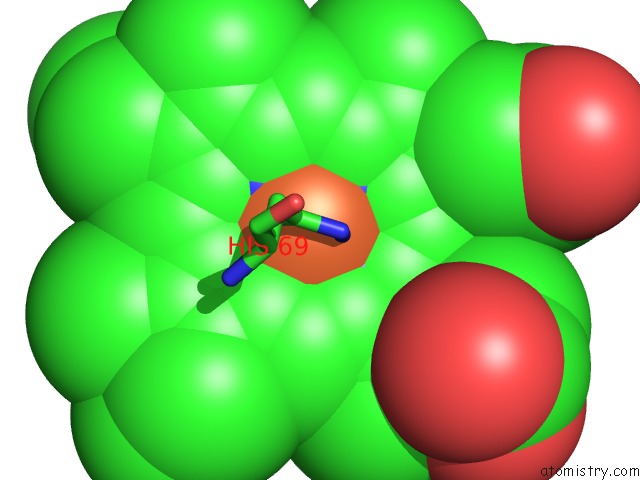
Mono view
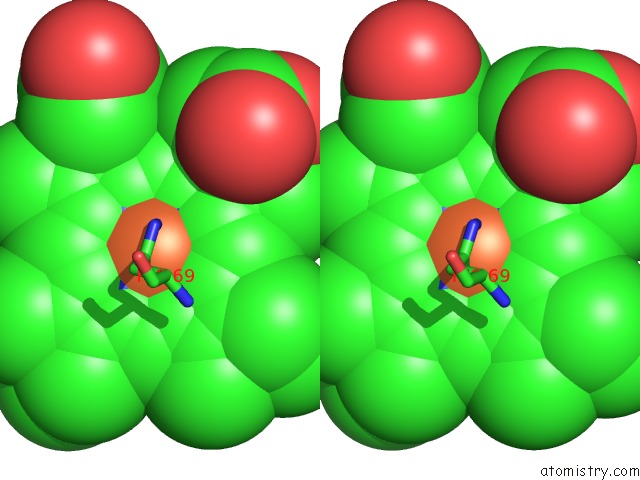
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cytochrome CD1 Nitrite Reductase, Oxidised Form within 5.0Å range:
|
Iron binding site 2 out of 4 in 1qks
Go back to
Iron binding site 2 out
of 4 in the Cytochrome CD1 Nitrite Reductase, Oxidised Form
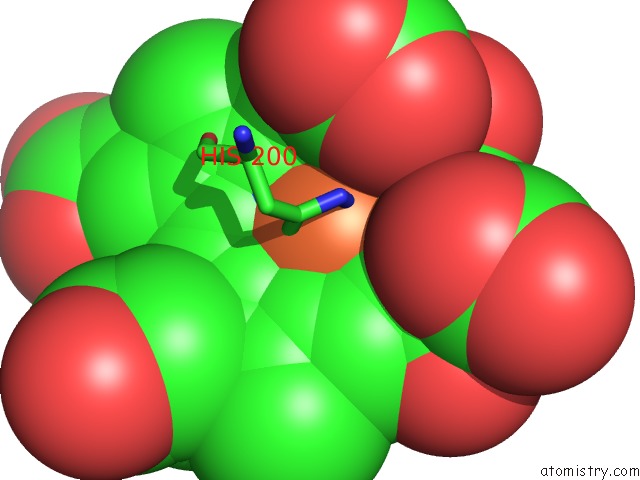
Mono view
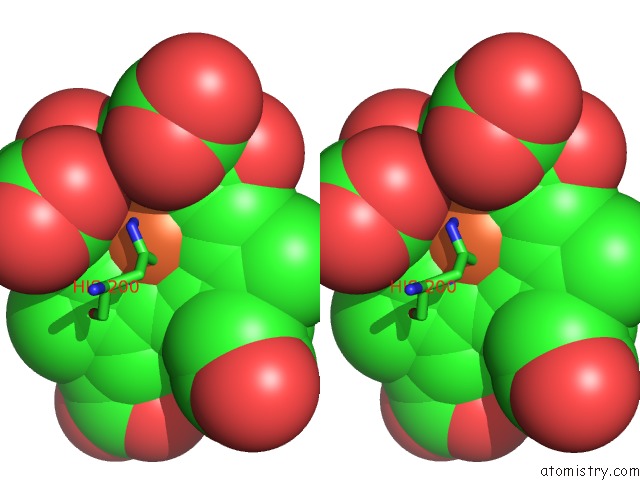
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cytochrome CD1 Nitrite Reductase, Oxidised Form within 5.0Å range:
|
Iron binding site 3 out of 4 in 1qks
Go back to
Iron binding site 3 out
of 4 in the Cytochrome CD1 Nitrite Reductase, Oxidised Form
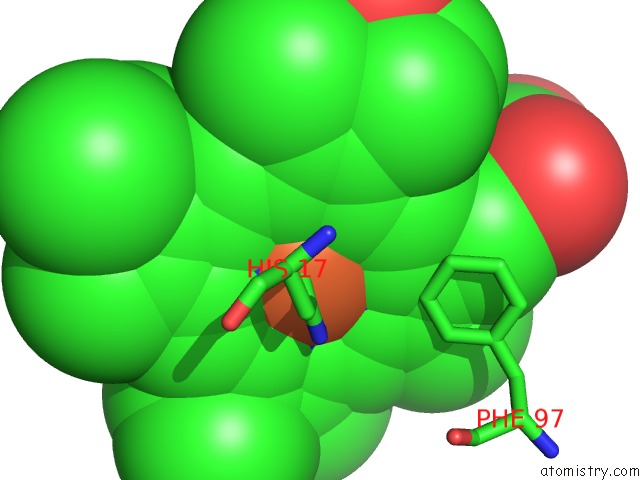
Mono view
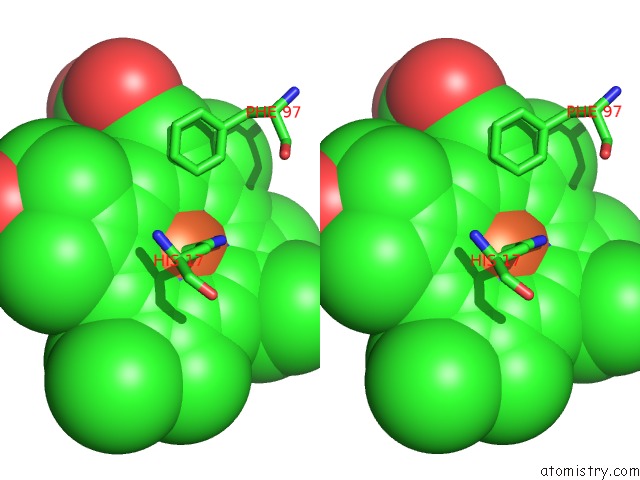
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cytochrome CD1 Nitrite Reductase, Oxidised Form within 5.0Å range:
|
Iron binding site 4 out of 4 in 1qks
Go back to
Iron binding site 4 out
of 4 in the Cytochrome CD1 Nitrite Reductase, Oxidised Form
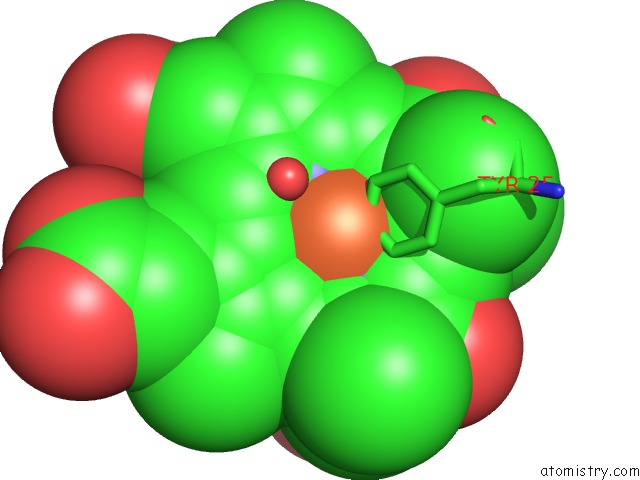
Mono view
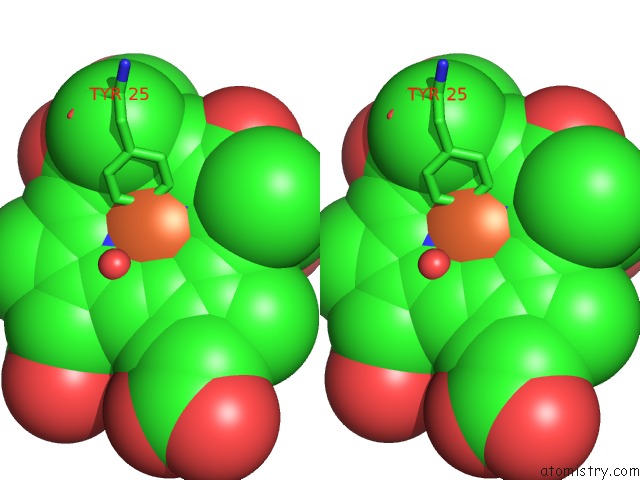
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cytochrome CD1 Nitrite Reductase, Oxidised Form within 5.0Å range:
|
Reference:
V.Fulop,
J.W.Moir,
S.J.Ferguson,
J.Hajdu.
The Anatomy of A Bifunctional Enzyme: Structural Basis For Reduction of Oxygen to Water and Synthesis of Nitric Oxide By Cytochrome CD1. Cell(Cambridge,Mass.) V. 81 369 1995.
ISSN: ISSN 0092-8674
PubMed: 7736589
DOI: 10.1016/0092-8674(95)90390-9
Page generated: Wed Jul 16 19:57:29 2025
ISSN: ISSN 0092-8674
PubMed: 7736589
DOI: 10.1016/0092-8674(95)90390-9
Last articles
Mg in 4QFYMg in 4QFX
Mg in 4QBY
Mg in 4QF5
Mg in 4QFM
Mg in 4QEH
Mg in 4QEL
Mg in 4QE5
Mg in 4QDG
Mg in 4QDM