Iron »
PDB 2ays-2bmm »
2bc5 »
Iron in PDB 2bc5: Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages
Protein crystallography data
The structure of Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages, PDB code: 2bc5
was solved by
J.Faraone-Mennella,
F.A.Tezcan,
H.B.Gray,
J.R.Winkler,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.40 / 2.25 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 63.861, 68.158, 90.440, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23.9 / 27.7 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages
(pdb code 2bc5). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages, PDB code: 2bc5:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages, PDB code: 2bc5:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 2bc5
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages within 5.0Å range:
|
Iron binding site 2 out of 4 in 2bc5
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages
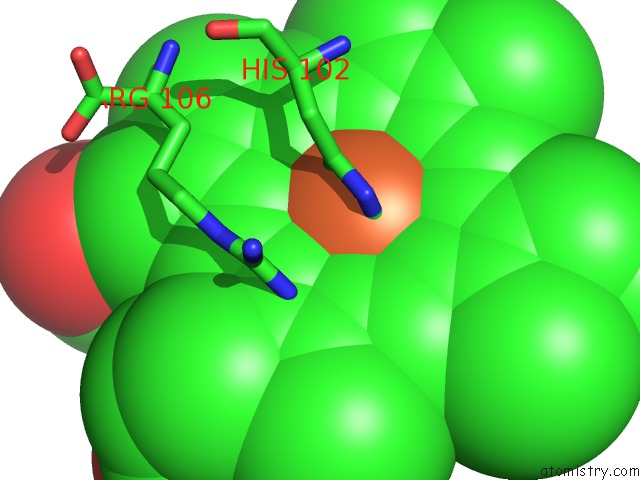
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages within 5.0Å range:
|
Iron binding site 3 out of 4 in 2bc5
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages within 5.0Å range:
|
Iron binding site 4 out of 4 in 2bc5
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of E. Coli Cytochrome B562 with Engineered C-Type Heme Linkages within 5.0Å range:
|
Reference:
J.Faraone-Mennella,
F.A.Tezcan,
H.B.Gray,
J.R.Winkler.
Stability and Folding Kinetics of Structurally Characterized Cytochrome C-B(562). Biochemistry V. 45 10504 2006.
ISSN: ISSN 0006-2960
PubMed: 16939202
DOI: 10.1021/BI060242X
Page generated: Wed Jul 16 23:50:57 2025
ISSN: ISSN 0006-2960
PubMed: 16939202
DOI: 10.1021/BI060242X
Last articles
Mg in 6CTAMg in 6CRH
Mg in 6CT2
Mg in 6CRC
Mg in 6CR9
Mg in 6CRB
Mg in 6CR8
Mg in 6CR7
Mg in 6CR5
Mg in 6CR4