Iron »
PDB 2ciz-2d3q »
2cmn »
Iron in PDB 2cmn: A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor
Enzymatic activity of A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor
All present enzymatic activity of A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor:
2.7.13.3;
2.7.13.3;
Protein crystallography data
The structure of A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor, PDB code: 2cmn
was solved by
M.-A.Gilles-Gonzalez,
A.I.Caceres,
E.H.Silva Sousa,
D.R.Tomchick,
C.A.Brautigam,
C.Gonzalez,
M.Machius,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.30 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 127.117, 127.117, 58.569, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.5 / 22 |
Iron Binding Sites:
The binding sites of Iron atom in the A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor
(pdb code 2cmn). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor, PDB code: 2cmn:
In total only one binding site of Iron was determined in the A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor, PDB code: 2cmn:
Iron binding site 1 out of 1 in 2cmn
Go back to
Iron binding site 1 out
of 1 in the A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor
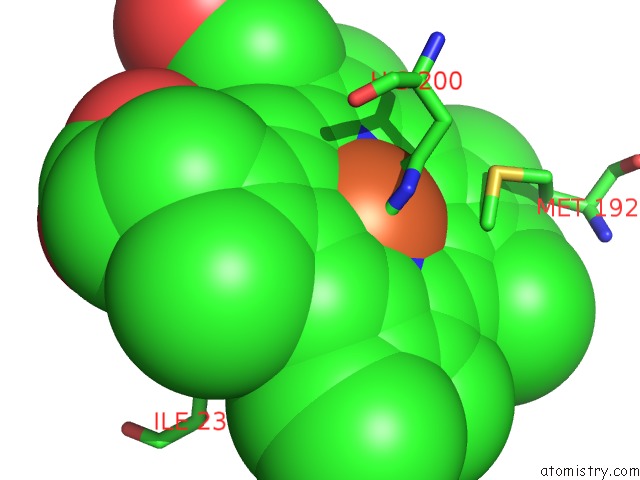
Mono view
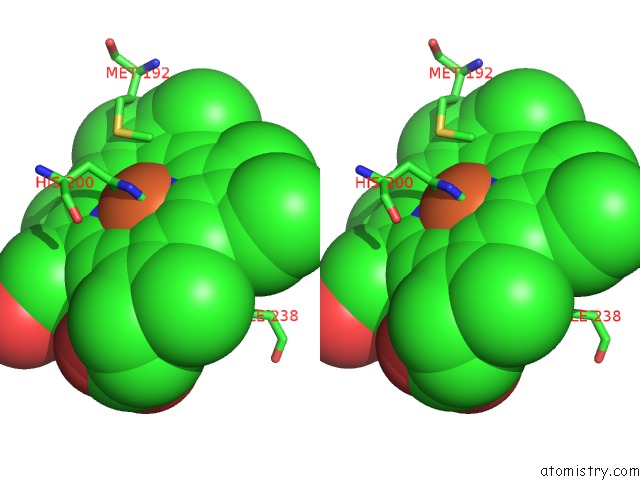
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of A Proximal Arginine Residue in the Switching Mechanism of the Fixl Oxygen Sensor within 5.0Å range:
|
Reference:
M.-A.Gilles-Gonzalez,
A.I.Caceres,
E.H.Silva Sousa,
D.R.Tomchick,
C.A.Brautigam,
C.Gonzalez,
M.Machius.
A Proximal Arginine R206 Participates in Switching of the Bradyrhizobium Japonicum Fixl Oxygen Sensor J.Mol.Biol. V. 360 80 2006.
ISSN: ISSN 0022-2836
PubMed: 16813836
DOI: 10.1016/J.JMB.2006.04.054
Page generated: Thu Jul 17 00:28:41 2025
ISSN: ISSN 0022-2836
PubMed: 16813836
DOI: 10.1016/J.JMB.2006.04.054
Last articles
Na in 3PPYNa in 3PPX
Na in 3PPW
Na in 3PO4
Na in 3PNC
Na in 3PO1
Na in 3PML
Na in 3PMN
Na in 3PM8
Na in 3PMB