Iron »
PDB 3dcp-3e0f »
3de8 »
Iron in PDB 3de8: Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination
Protein crystallography data
The structure of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination, PDB code: 3de8
was solved by
E.N.Salgado,
R.A.Lewis,
A.L.Rheingold,
F.A.Tezcan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 32.92 / 1.72 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 47.566, 89.504, 52.019, 90.00, 111.01, 90.00 |
| R / Rfree (%) | 19.4 / 24.4 |
Other elements in 3de8:
The structure of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination also contains other interesting chemical elements:
| Copper | (Cu) | 4 atoms |
| Calcium | (Ca) | 6 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination
(pdb code 3de8). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination, PDB code: 3de8:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination, PDB code: 3de8:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 3de8
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination within 5.0Å range:
|
Iron binding site 2 out of 4 in 3de8
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination
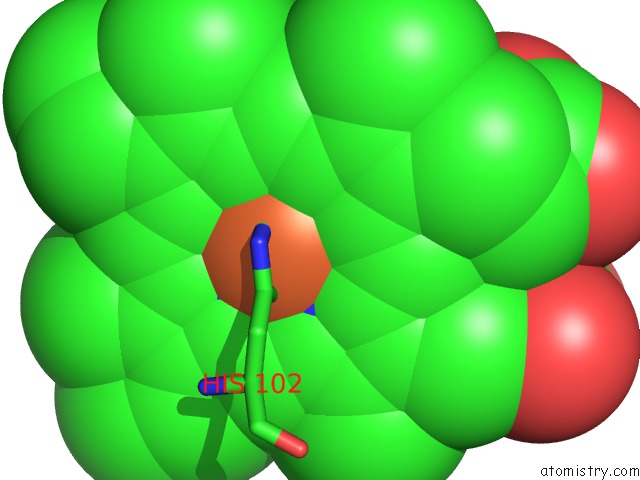
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination within 5.0Å range:
|
Iron binding site 3 out of 4 in 3de8
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination
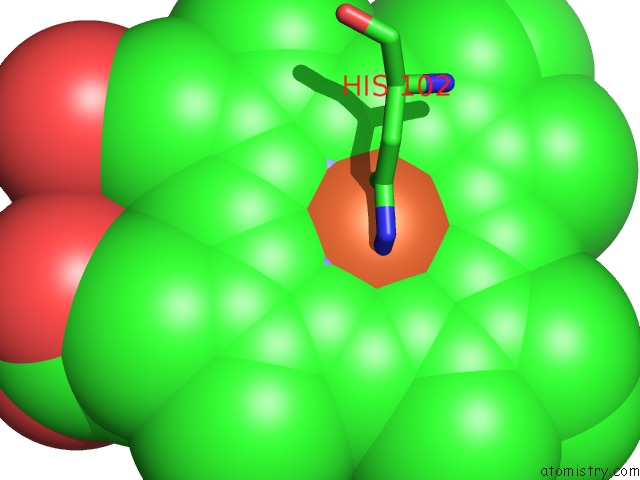
Mono view
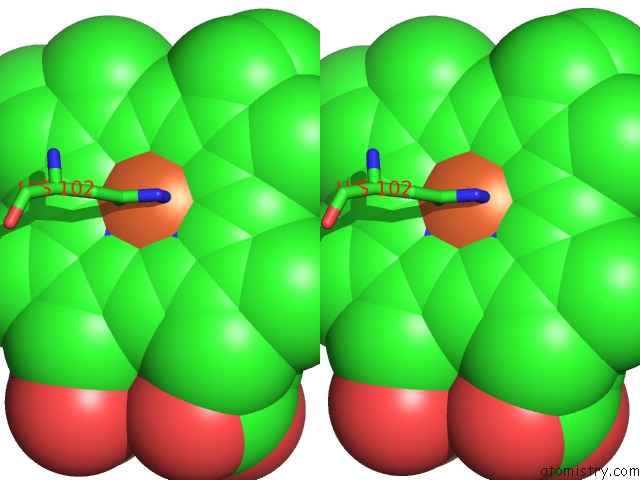
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination within 5.0Å range:
|
Iron binding site 4 out of 4 in 3de8
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of A Dimeric Cytochrome CB562 Assembly Induced By Copper Coordination within 5.0Å range:
|
Reference:
E.N.Salgado,
R.A.Lewis,
S.Mossin,
A.L.Rheingold,
F.A.Tezcan.
Control of Protein Oligomerization Symmetry By Metal Coordination: C2 and C3 Symmetrical Assemblies Through Cu(II) and Ni(II) Coordination. Inorg.Chem. V. 48 2726 2009.
ISSN: ISSN 0020-1669
PubMed: 19267481
DOI: 10.1021/IC9001237
Page generated: Tue Aug 5 00:36:05 2025
ISSN: ISSN 0020-1669
PubMed: 19267481
DOI: 10.1021/IC9001237
Last articles
I in 5DL9I in 5DJC
I in 5D69
I in 5DAM
I in 5CW1
I in 5D55
I in 5CNK
I in 5CQF
I in 5CMW
I in 5CR1