Iron »
PDB 3s6b-3sxt »
3sid »
Iron in PDB 3sid: Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
Protein crystallography data
The structure of Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy, PDB code: 3sid
was solved by
R.B.Cooley,
D.J.Arp,
P.A.Karplus,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.74 / 1.40 |
| Space group | P 32 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.009, 82.009, 46.440, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 11.5 / 15.6 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
(pdb code 3sid). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy, PDB code: 3sid:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy, PDB code: 3sid:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 3sid
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
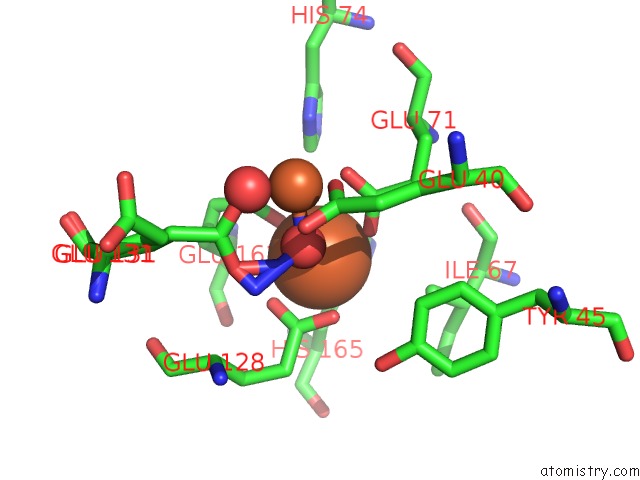
Mono view
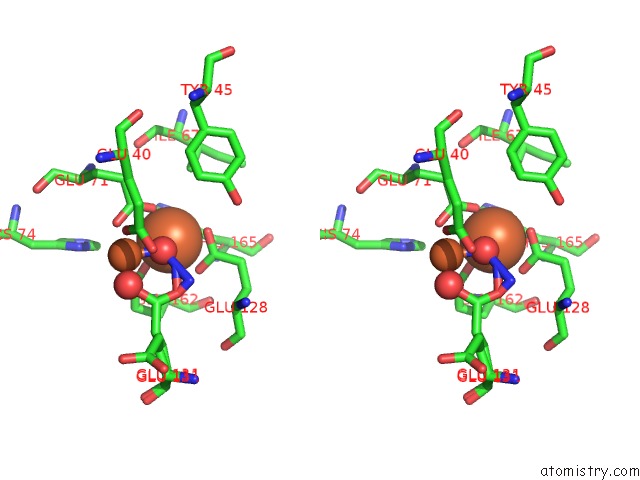
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy within 5.0Å range:
|
Iron binding site 2 out of 4 in 3sid
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
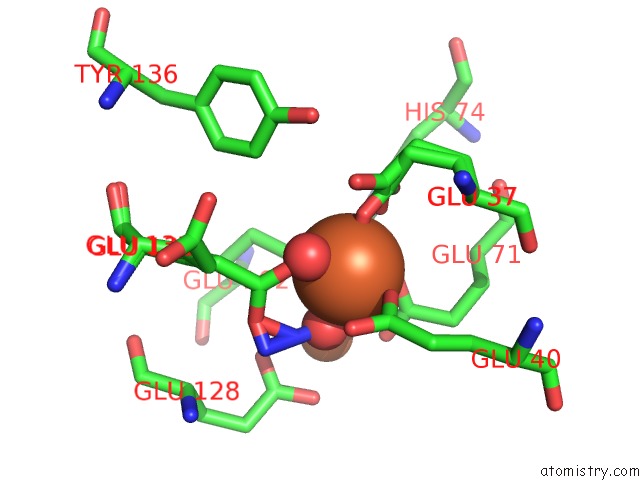
Mono view
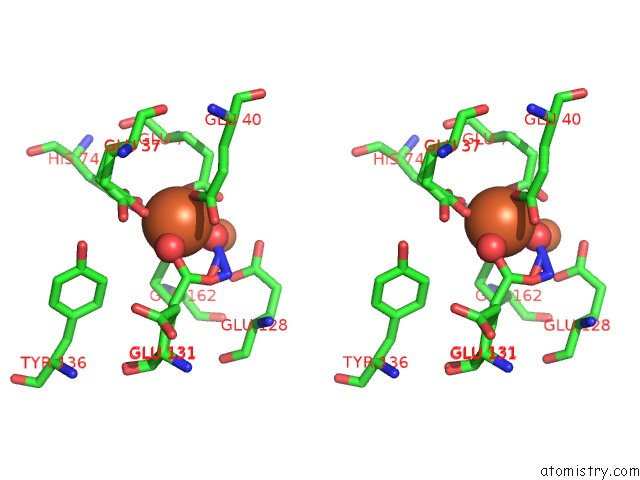
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy within 5.0Å range:
|
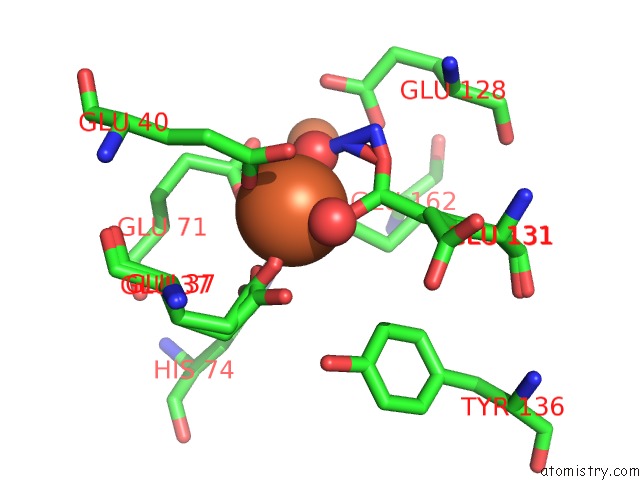
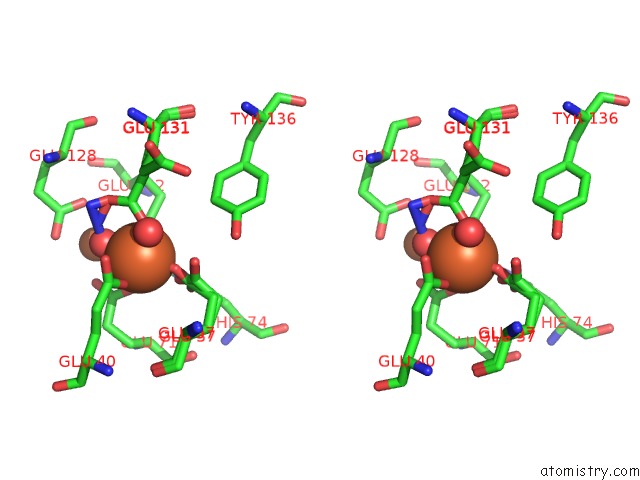
Iron binding site 3 out of 4 in 3sid
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
Mono view
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy within 5.0Å range:
|
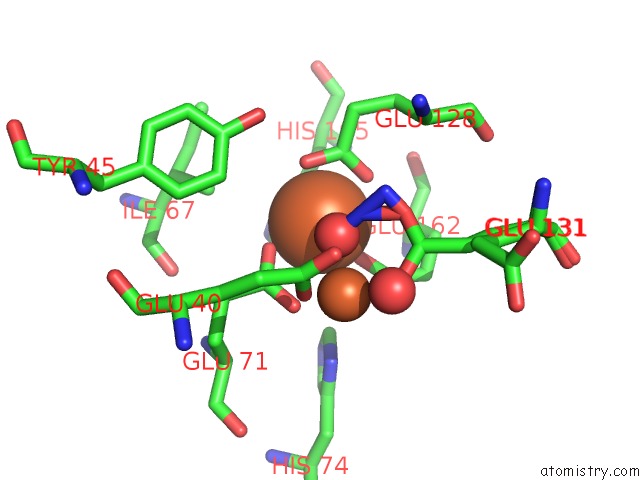
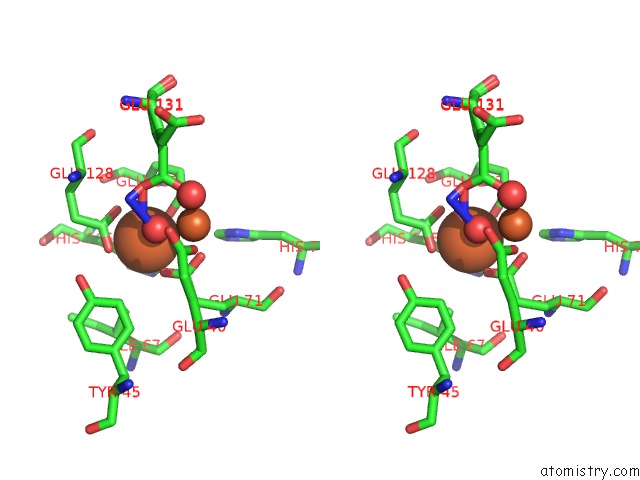
Iron binding site 4 out of 4 in 3sid
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy
Mono view
Stereo pair view

Mono view

Stereo pair view
|
|
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of Oxidized Symerythrin From Cyanophora Paradoxa, Azide Adduct at 50% Occupancy within 5.0Å range:
|
Reference:
R.B.Cooley,
D.J.Arp,
P.A.Karplus.
Symerythrin Structures at Atomic Resolution and the Origins of Rubrerythrins and the Ferritin-Like Superfamily. J.Mol.Biol. V. 413 177 2011.
ISSN: ISSN 0022-2836
PubMed: 21872605
DOI: 10.1016/J.JMB.2011.08.019
Page generated: Tue Aug 5 06:45:28 2025
ISSN: ISSN 0022-2836
PubMed: 21872605
DOI: 10.1016/J.JMB.2011.08.019
Last articles
Zn in 2B3ZZn in 2B5W
Zn in 2B3J
Zn in 2B44
Zn in 2B13
Zn in 2B3X
Zn in 2AYI
Zn in 2B11
Zn in 2B12
Zn in 2B10