Iron »
PDB 4gl7-4h9l »
4h24 »
Iron in PDB 4h24: Cytochrome P450BM3-Cis Cyclopropanation Catalyst
Enzymatic activity of Cytochrome P450BM3-Cis Cyclopropanation Catalyst
All present enzymatic activity of Cytochrome P450BM3-Cis Cyclopropanation Catalyst:
1.14.14.1;
1.14.14.1;
Protein crystallography data
The structure of Cytochrome P450BM3-Cis Cyclopropanation Catalyst, PDB code: 4h24
was solved by
P.S.Coelho,
Z.J.Wang,
M.E.Ener,
S.A.Baril,
A.Kannan,
F.H.Arnold,
E.M.Brustad,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.58 / 2.50 |
| Space group | I 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 187.792, 62.745, 210.278, 90.00, 115.75, 90.00 |
| R / Rfree (%) | 18.4 / 24.7 |
Iron Binding Sites:
The binding sites of Iron atom in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst
(pdb code 4h24). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst, PDB code: 4h24:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst, PDB code: 4h24:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 4h24
Go back to
Iron binding site 1 out
of 4 in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst
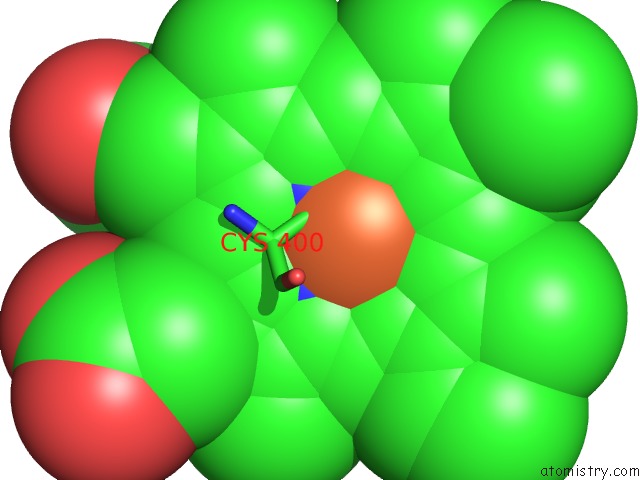
Mono view
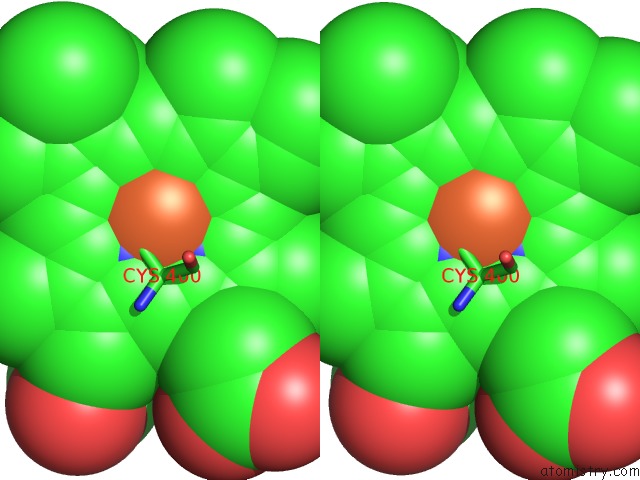
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cytochrome P450BM3-Cis Cyclopropanation Catalyst within 5.0Å range:
|
Iron binding site 2 out of 4 in 4h24
Go back to
Iron binding site 2 out
of 4 in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cytochrome P450BM3-Cis Cyclopropanation Catalyst within 5.0Å range:
|
Iron binding site 3 out of 4 in 4h24
Go back to
Iron binding site 3 out
of 4 in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst

Mono view
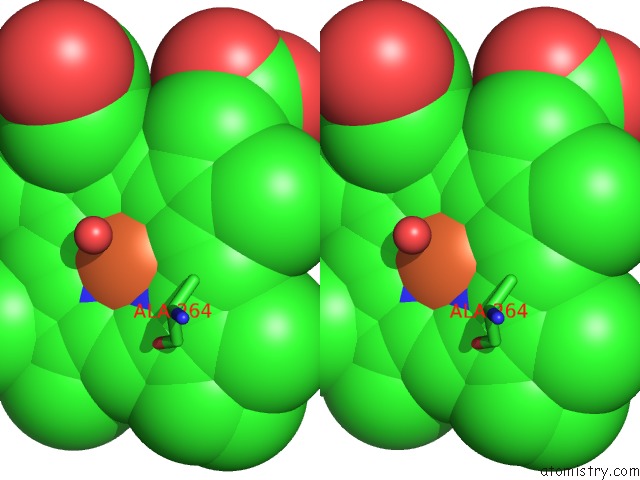
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cytochrome P450BM3-Cis Cyclopropanation Catalyst within 5.0Å range:
|
Iron binding site 4 out of 4 in 4h24
Go back to
Iron binding site 4 out
of 4 in the Cytochrome P450BM3-Cis Cyclopropanation Catalyst
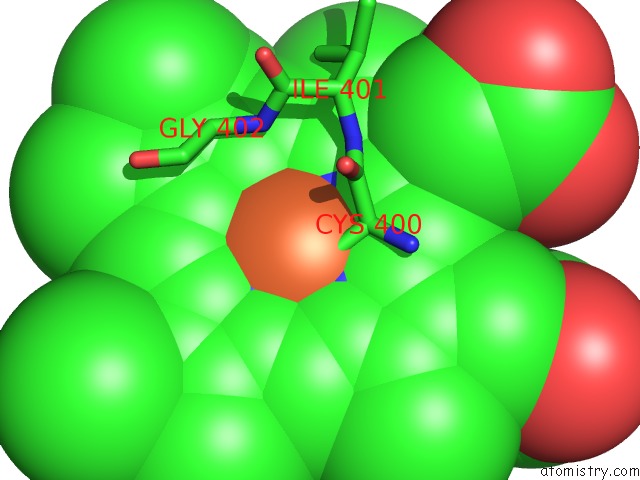
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cytochrome P450BM3-Cis Cyclopropanation Catalyst within 5.0Å range:
|
Reference:
P.S.Coelho,
Z.J.Wang,
M.E.Ener,
S.A.Baril,
A.Kannan,
F.H.Arnold,
E.M.Brustad.
A Serine-Substituted P450 Catalyzes Highly Efficient Carbene Transfer to Olefins in Vivo. Nat.Chem.Biol. V. 9 485 2013.
ISSN: ISSN 1552-4450
PubMed: 23792734
DOI: 10.1038/NCHEMBIO.1278
Page generated: Tue Aug 5 10:49:42 2025
ISSN: ISSN 1552-4450
PubMed: 23792734
DOI: 10.1038/NCHEMBIO.1278
Last articles
Mg in 5MM7Mg in 5ML7
Mg in 5MM9
Mg in 5MLB
Mg in 5MM4
Mg in 5MLZ
Mg in 5MLA
Mg in 5MIL
Mg in 5MIO
Mg in 5MHA