Iron »
PDB 4jo0-4k5d »
4k19 »
Iron in PDB 4k19: The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin
Protein crystallography data
The structure of The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin, PDB code: 4k19
was solved by
C.Correnti,
M.C.Clifton,
R.K.Strong,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.95 / 2.74 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.200, 114.200, 119.300, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23.6 / 29.4 |
Other elements in 4k19:
The structure of The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
| Sodium | (Na) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin
(pdb code 4k19). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 3 binding sites of Iron where determined in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin, PDB code: 4k19:
Jump to Iron binding site number: 1; 2; 3;
In total 3 binding sites of Iron where determined in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin, PDB code: 4k19:
Jump to Iron binding site number: 1; 2; 3;
Iron binding site 1 out of 3 in 4k19
Go back to
Iron binding site 1 out
of 3 in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin
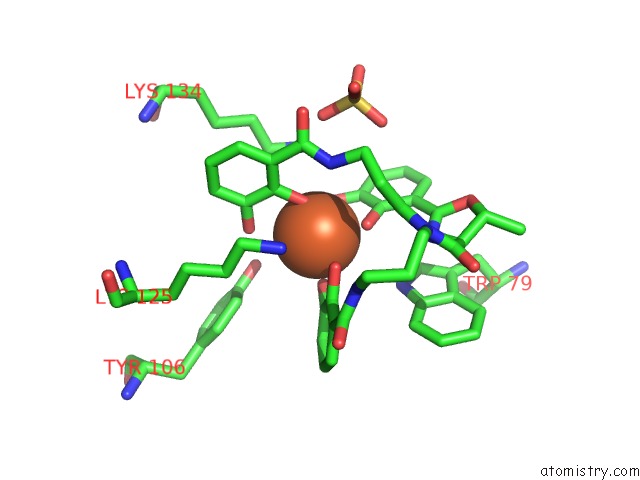
Mono view
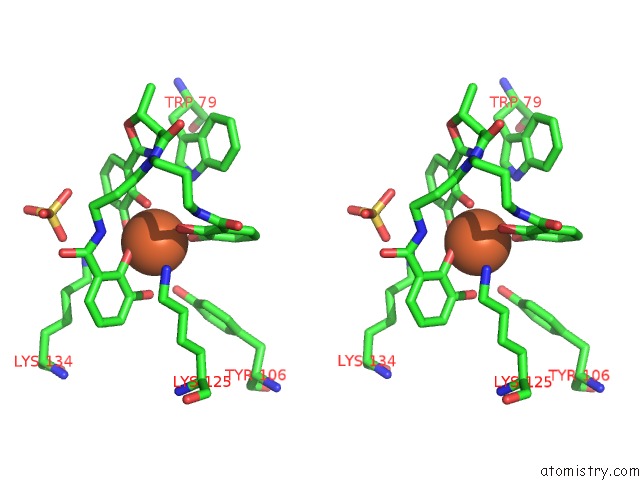
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin within 5.0Å range:
|
Iron binding site 2 out of 3 in 4k19
Go back to
Iron binding site 2 out
of 3 in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin
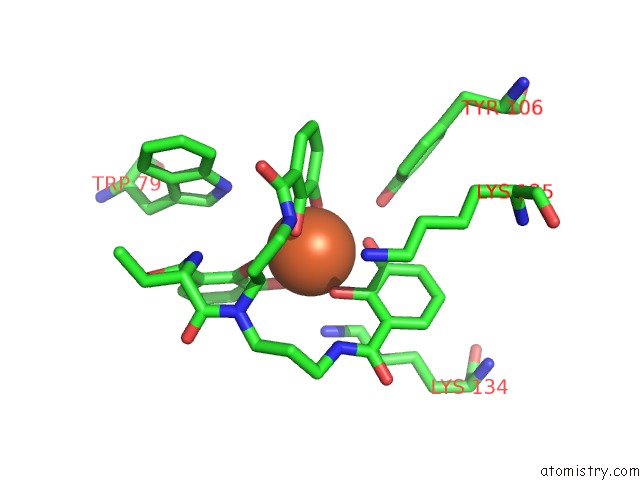
Mono view
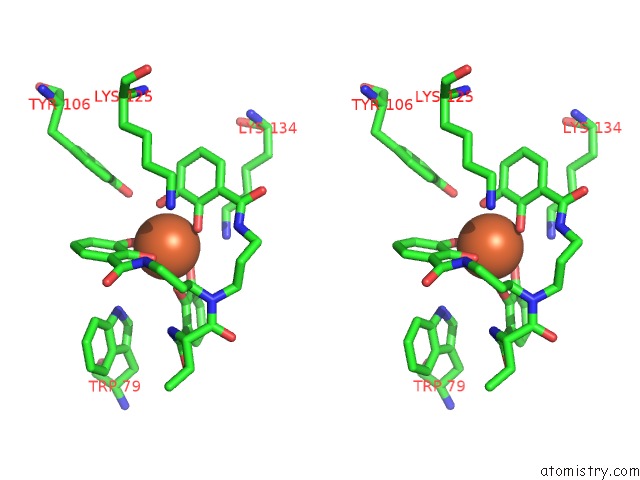
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin within 5.0Å range:
|
Iron binding site 3 out of 3 in 4k19
Go back to
Iron binding site 3 out
of 3 in the The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin
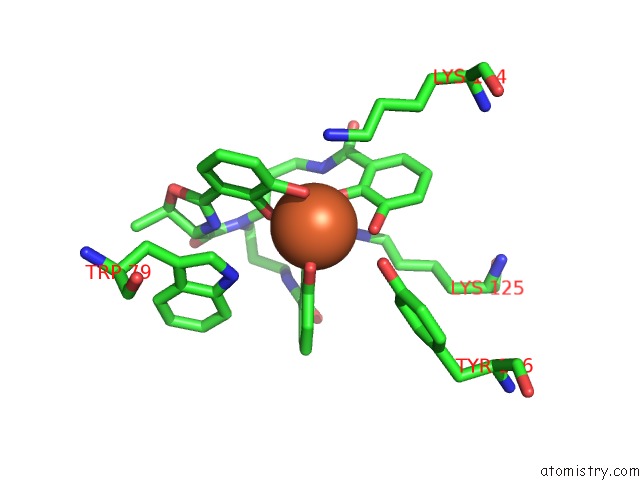
Mono view
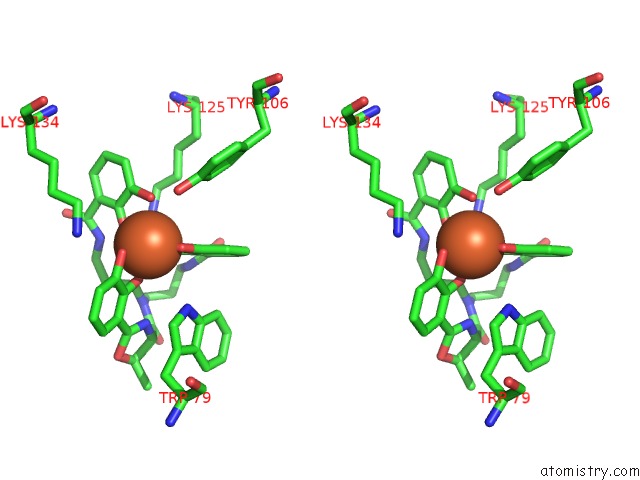
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of The Structure of Human Siderocalin Bound to the Bacterial Siderophore Fluvibactin within 5.0Å range:
|
Reference:
B.E.Allred,
C.Correnti,
M.C.Clifton,
R.K.Strong,
K.N.Raymond.
Siderocalin Outwits the Coordination Chemistry of Vibriobactin, A Siderophore of Vibrio Cholerae. Acs Chem.Biol. V. 8 1882 2013.
ISSN: ISSN 1554-8929
PubMed: 23755875
DOI: 10.1021/CB4002552
Page generated: Tue Aug 5 11:44:38 2025
ISSN: ISSN 1554-8929
PubMed: 23755875
DOI: 10.1021/CB4002552
Last articles
Sr in 3BNQSr in 2QJY
Sr in 2X53
Sr in 2SPT
Sr in 2XRM
Sr in 2WOH
Sr in 2RIO
Sr in 2PN4
Sr in 2QJK
Sr in 2QJP