Iron »
PDB 5c2i-5cnd »
5ci1 »
Iron in PDB 5ci1: Ribonucleotide Reductase Y122 2,3-F2Y Variant
Enzymatic activity of Ribonucleotide Reductase Y122 2,3-F2Y Variant
All present enzymatic activity of Ribonucleotide Reductase Y122 2,3-F2Y Variant:
1.17.4.1;
1.17.4.1;
Protein crystallography data
The structure of Ribonucleotide Reductase Y122 2,3-F2Y Variant, PDB code: 5ci1
was solved by
M.A.Funk,
C.L.Drennan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.00 / 1.95 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 90.589, 90.589, 209.468, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 15.4 / 18.3 |
Other elements in 5ci1:
The structure of Ribonucleotide Reductase Y122 2,3-F2Y Variant also contains other interesting chemical elements:
| Fluorine | (F) | 4 atoms |
| Chlorine | (Cl) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Ribonucleotide Reductase Y122 2,3-F2Y Variant
(pdb code 5ci1). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Ribonucleotide Reductase Y122 2,3-F2Y Variant, PDB code: 5ci1:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Ribonucleotide Reductase Y122 2,3-F2Y Variant, PDB code: 5ci1:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 5ci1
Go back to
Iron binding site 1 out
of 2 in the Ribonucleotide Reductase Y122 2,3-F2Y Variant
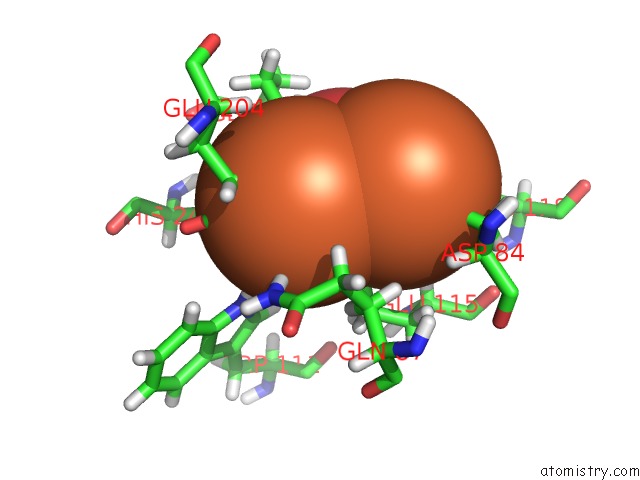
Mono view
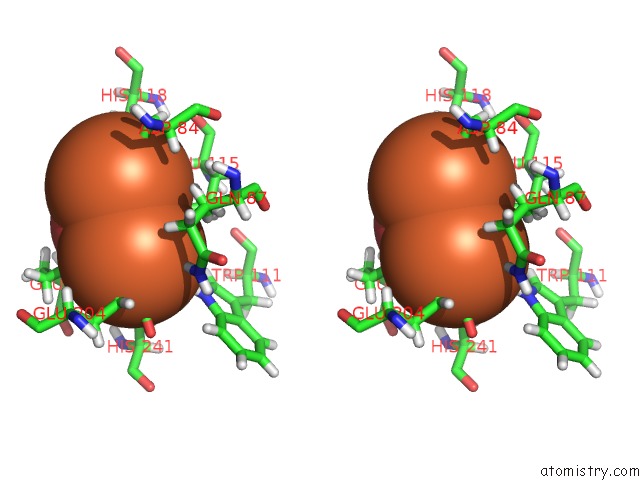
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Ribonucleotide Reductase Y122 2,3-F2Y Variant within 5.0Å range:
|
Iron binding site 2 out of 2 in 5ci1
Go back to
Iron binding site 2 out
of 2 in the Ribonucleotide Reductase Y122 2,3-F2Y Variant
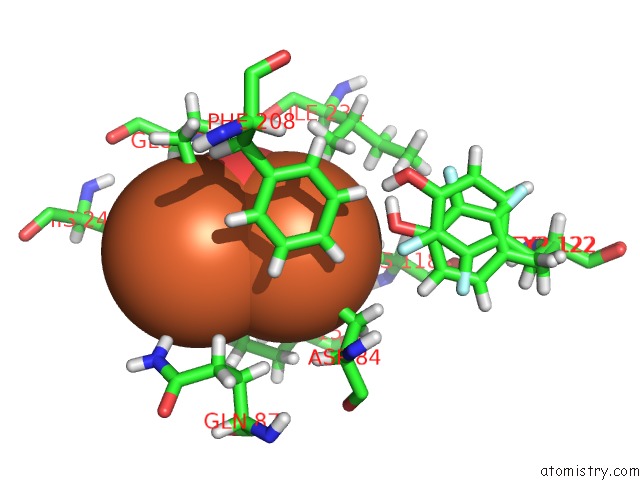
Mono view
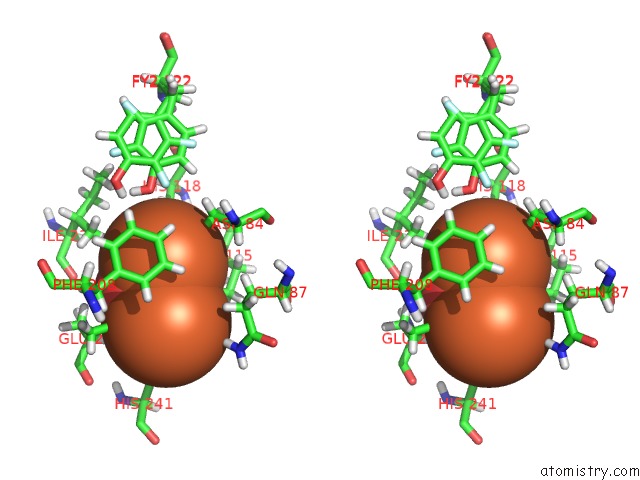
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Ribonucleotide Reductase Y122 2,3-F2Y Variant within 5.0Å range:
|
Reference:
P.H.Oyala,
K.R.Ravichandran,
M.A.Funk,
P.A.Stucky,
T.A.Stich,
C.L.Drennan,
R.D.Britt,
J.Stubbe.
Biophysical Characterization of Fluorotyrosine Probes Site-Specifically Incorporated Into Enzymes: E. Coli Ribonucleotide Reductase As An Example. J.Am.Chem.Soc. V. 138 7951 2016.
ISSN: ESSN 1520-5126
PubMed: 27276098
DOI: 10.1021/JACS.6B03605
Page generated: Tue Aug 5 19:49:24 2025
ISSN: ESSN 1520-5126
PubMed: 27276098
DOI: 10.1021/JACS.6B03605
Last articles
K in 6SE7K in 6SE5
K in 6SBI
K in 6RVZ
K in 6S15
K in 6RW0
K in 6RWV
K in 6RV4
K in 6RV3
K in 6RV2