Iron »
PDB 5ug2-5utc »
5ul4 »
Iron in PDB 5ul4: Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
Protein crystallography data
The structure of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound, PDB code: 5ul4
was solved by
J.Bridwell-Rabb,
C.L.Drennan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.84 / 1.85 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.360, 99.600, 121.440, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.2 / 23.1 |
Other elements in 5ul4:
The structure of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound also contains other interesting chemical elements:
| Cobalt | (Co) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
(pdb code 5ul4). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound, PDB code: 5ul4:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound, PDB code: 5ul4:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 5ul4
Go back to
Iron binding site 1 out
of 4 in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
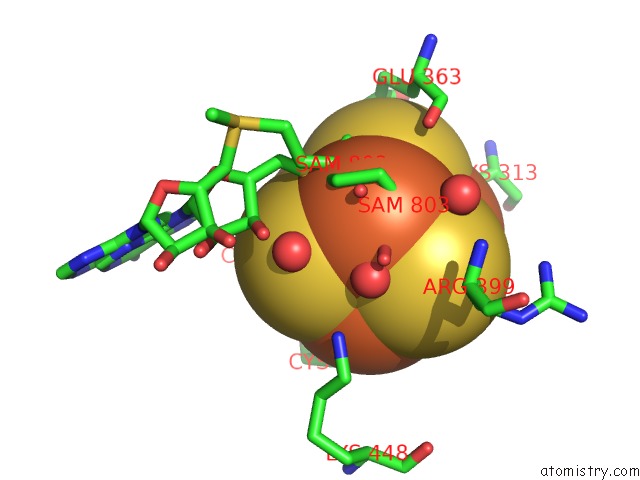
Mono view
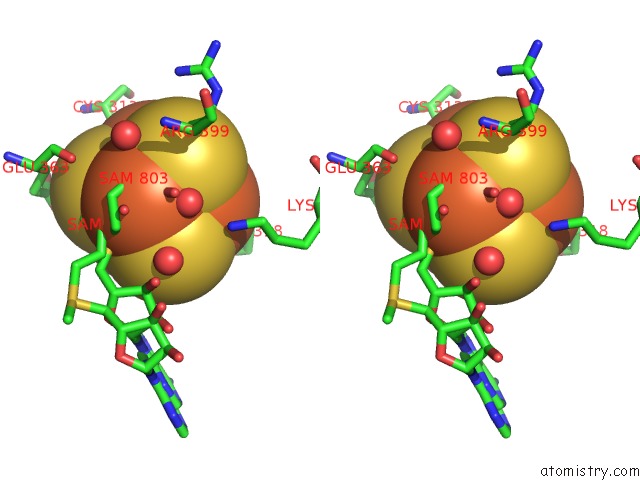
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound within 5.0Å range:
|
Iron binding site 2 out of 4 in 5ul4
Go back to
Iron binding site 2 out
of 4 in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
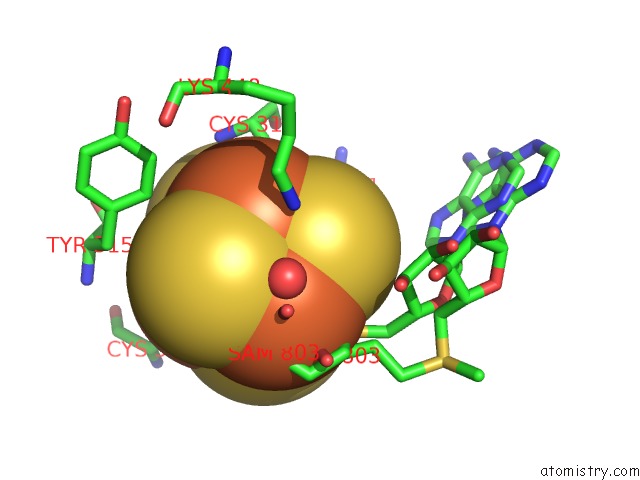
Mono view
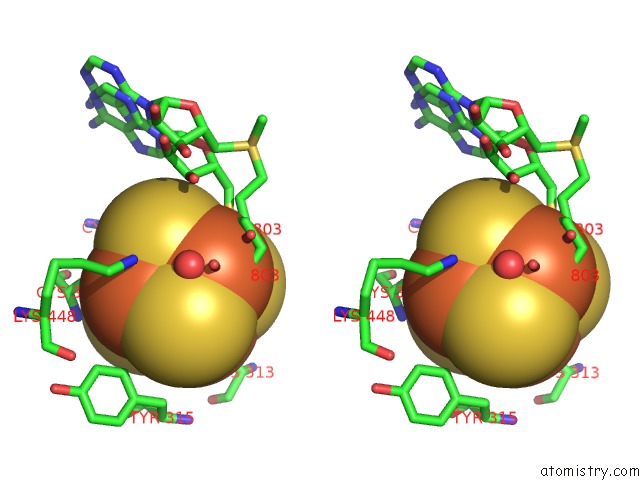
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound within 5.0Å range:
|
Iron binding site 3 out of 4 in 5ul4
Go back to
Iron binding site 3 out
of 4 in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
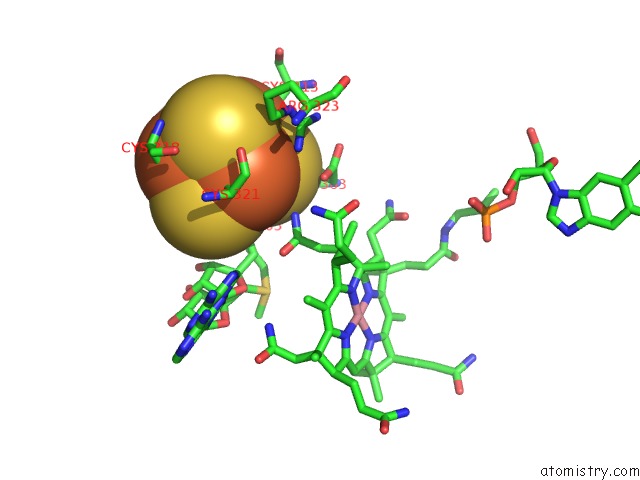
Mono view
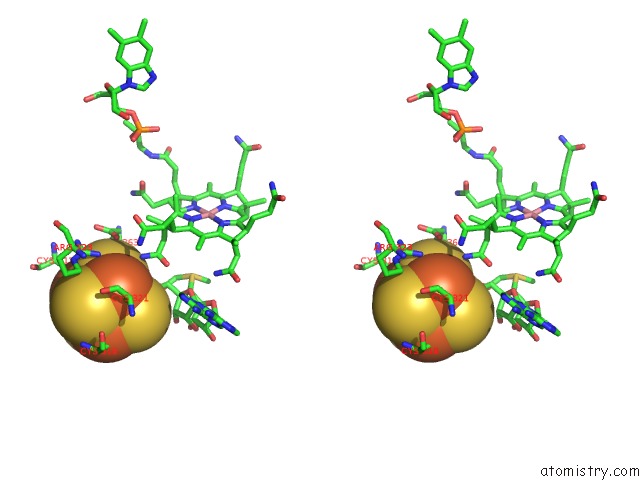
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound within 5.0Å range:
|
Iron binding site 4 out of 4 in 5ul4
Go back to
Iron binding site 4 out
of 4 in the Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound
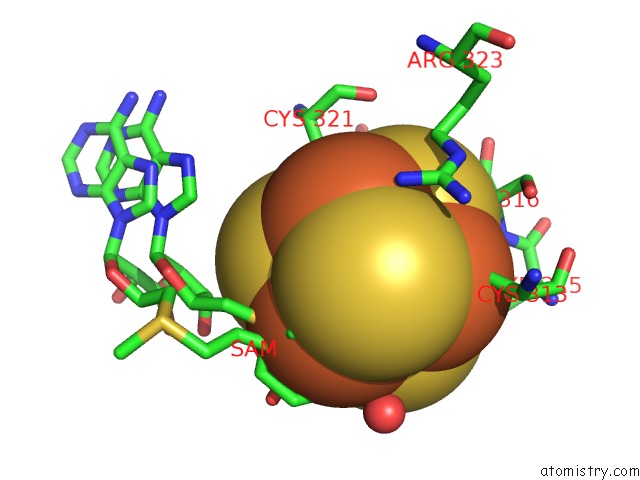
Mono view
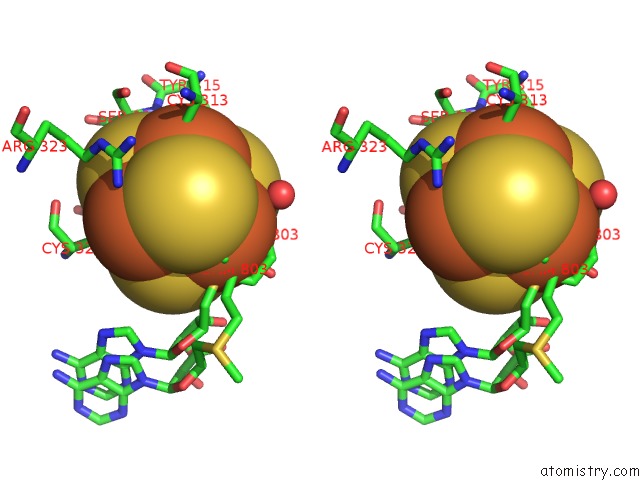
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of Cobalamin-Dependent S-Adenosylmethionine Radical Enzyme Oxsb with Aqua-Cobalamin and S-Adenosylmethionine Bound within 5.0Å range:
|
Reference:
J.Bridwell-Rabb,
A.Zhong,
H.G.Sun,
C.L.Drennan,
H.W.Liu.
A B12-Dependent Radical Sam Enzyme Involved in Oxetanocin A Biosynthesis. Nature V. 544 322 2017.
ISSN: ESSN 1476-4687
PubMed: 28346939
DOI: 10.1038/NATURE21689
Page generated: Wed Aug 6 01:53:03 2025
ISSN: ESSN 1476-4687
PubMed: 28346939
DOI: 10.1038/NATURE21689
Last articles
Na in 4TL5Na in 4RYF
Na in 4RU4
Na in 4S0N
Na in 4S2L
Na in 4RZR
Na in 4RZM
Na in 4RYD
Na in 4RU5
Na in 4RWD