Iron »
PDB 6ngi-6nlk »
6nhg »
Iron in PDB 6nhg: Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
Enzymatic activity of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
All present enzymatic activity of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex:
1.10.2.2;
1.10.2.2;
Protein crystallography data
The structure of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex, PDB code: 6nhg
was solved by
D.Xia,
F.Zhou,
L.Esser,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.87 / 2.80 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 154.181, 154.181, 598.180, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 25.1 / 28.9 |
Iron Binding Sites:
The binding sites of Iron atom in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
(pdb code 6nhg). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 5 binding sites of Iron where determined in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex, PDB code: 6nhg:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Iron where determined in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex, PDB code: 6nhg:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
Iron binding site 1 out of 5 in 6nhg
Go back to
Iron binding site 1 out
of 5 in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
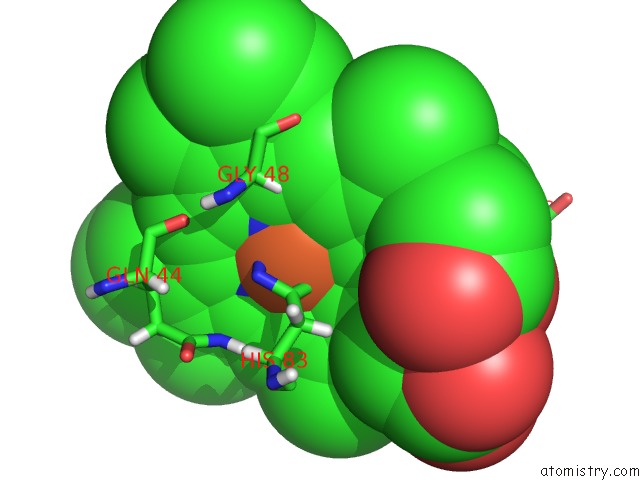
Mono view
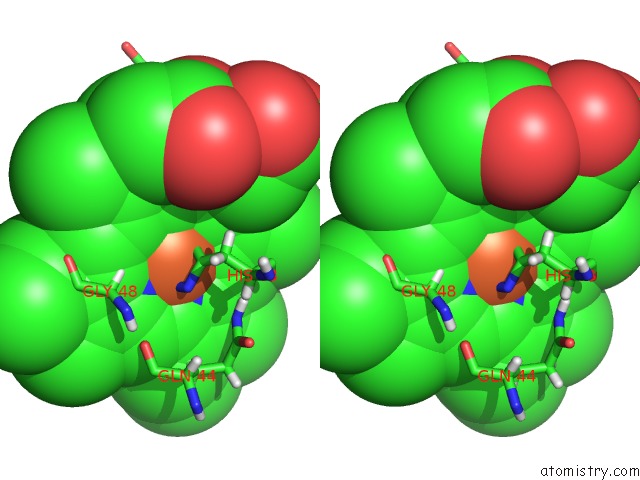
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex within 5.0Å range:
|
Iron binding site 2 out of 5 in 6nhg
Go back to
Iron binding site 2 out
of 5 in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
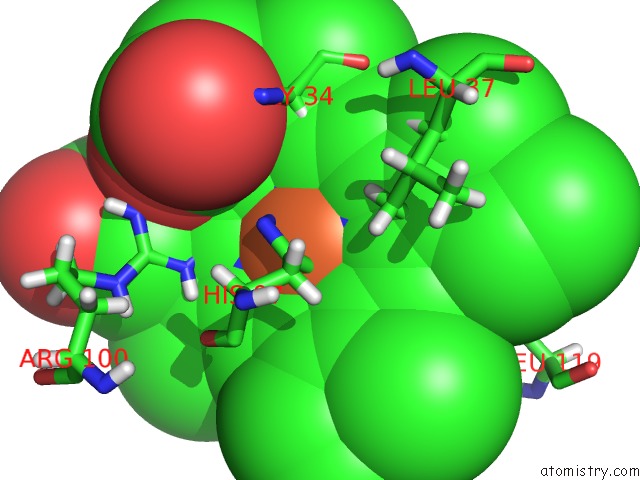
Mono view
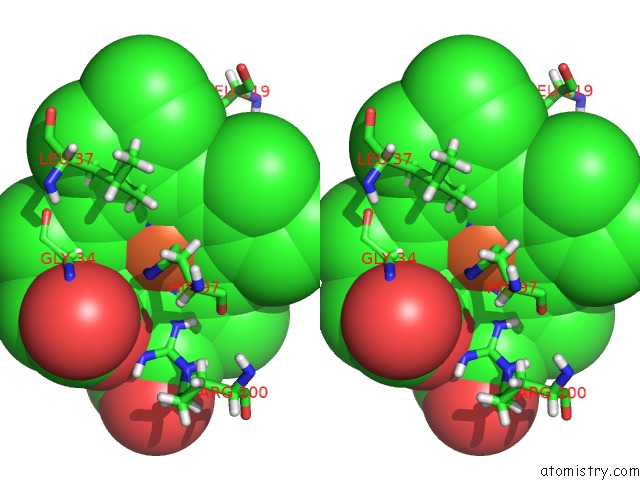
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex within 5.0Å range:
|
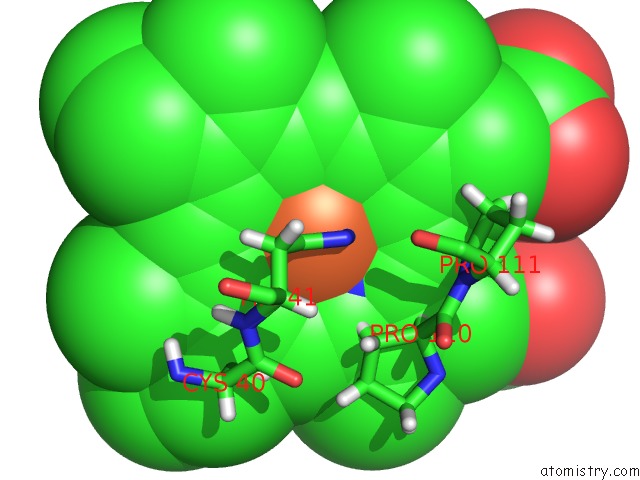
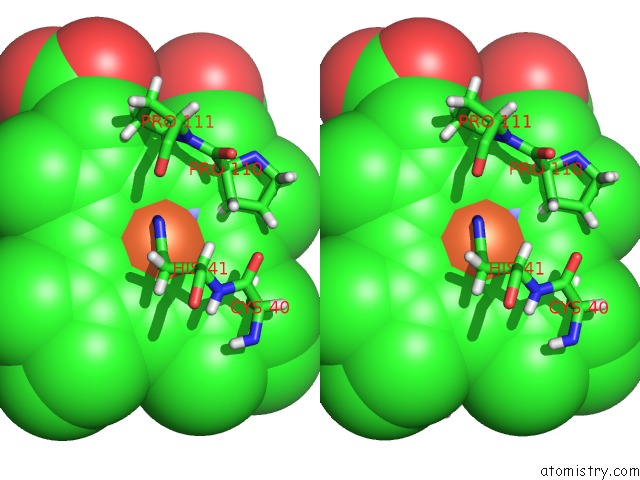
Iron binding site 3 out of 5 in 6nhg
Go back to
Iron binding site 3 out
of 5 in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex within 5.0Å range:
|
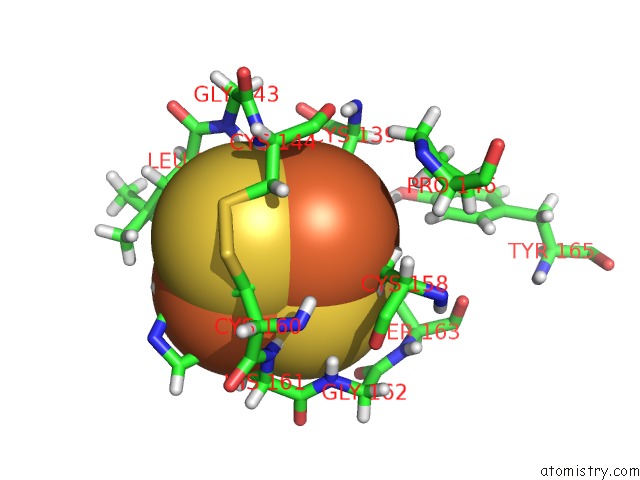
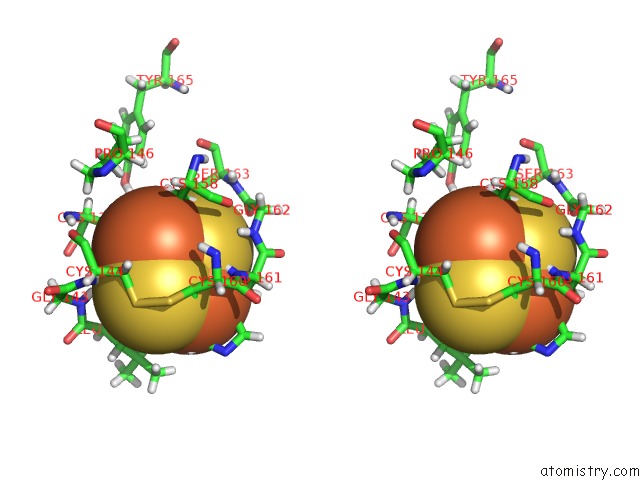
Iron binding site 4 out of 5 in 6nhg
Go back to
Iron binding site 4 out
of 5 in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex within 5.0Å range:
|
Iron binding site 5 out of 5 in 6nhg
Go back to
Iron binding site 5 out
of 5 in the Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex
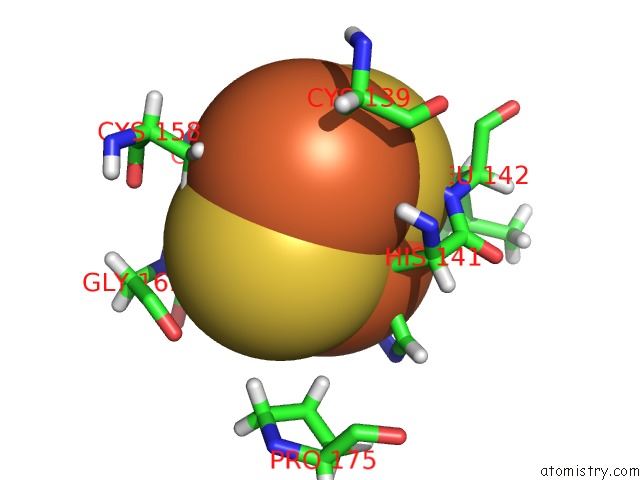
Mono view
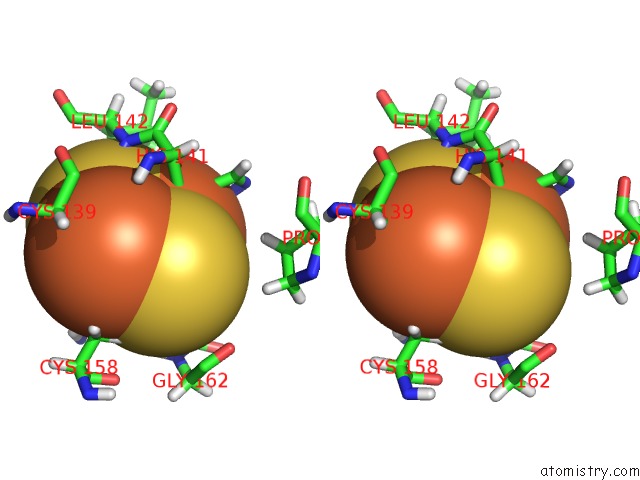
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Rhodobacter Sphaeroides Mitochondrial Respiratory Chain Complex within 5.0Å range:
|
Reference:
L.Esser,
F.Zhou,
C.A.Yu,
D.Xia.
Crystal Structure of Bacterial CYTOCHROMEBC1IN Complex with Azoxystrobin Reveals A Conformational Switch of the Rieske Iron-Sulfur Protein Subunit. J.Biol.Chem. V. 294 12007 2019.
ISSN: ESSN 1083-351X
PubMed: 31182483
DOI: 10.1074/JBC.RA119.008381
Page generated: Wed Aug 6 10:26:07 2025
ISSN: ESSN 1083-351X
PubMed: 31182483
DOI: 10.1074/JBC.RA119.008381
Last articles
Fe in 7R2SFe in 7R0W
Fe in 7R2R
Fe in 7R2P
Fe in 7R2O
Fe in 7R1J
Fe in 7R1I
Fe in 7QWT
Fe in 7R1H
Fe in 7R0F