Iron »
PDB 6tny-6u97 »
6to2 »
Iron in PDB 6to2: Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One
Protein crystallography data
The structure of Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One, PDB code: 6to2
was solved by
A.Rodriguez,
T.Kluenemann,
W.Blankenfeldt,
A.Schallmey,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 82.85 / 2.00 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 103.411, 103.411, 218.210, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 20.7 / 24.9 |
Other elements in 6to2:
The structure of Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One
(pdb code 6to2). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One, PDB code: 6to2:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One, PDB code: 6to2:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 6to2
Go back to
Iron binding site 1 out
of 2 in the Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One
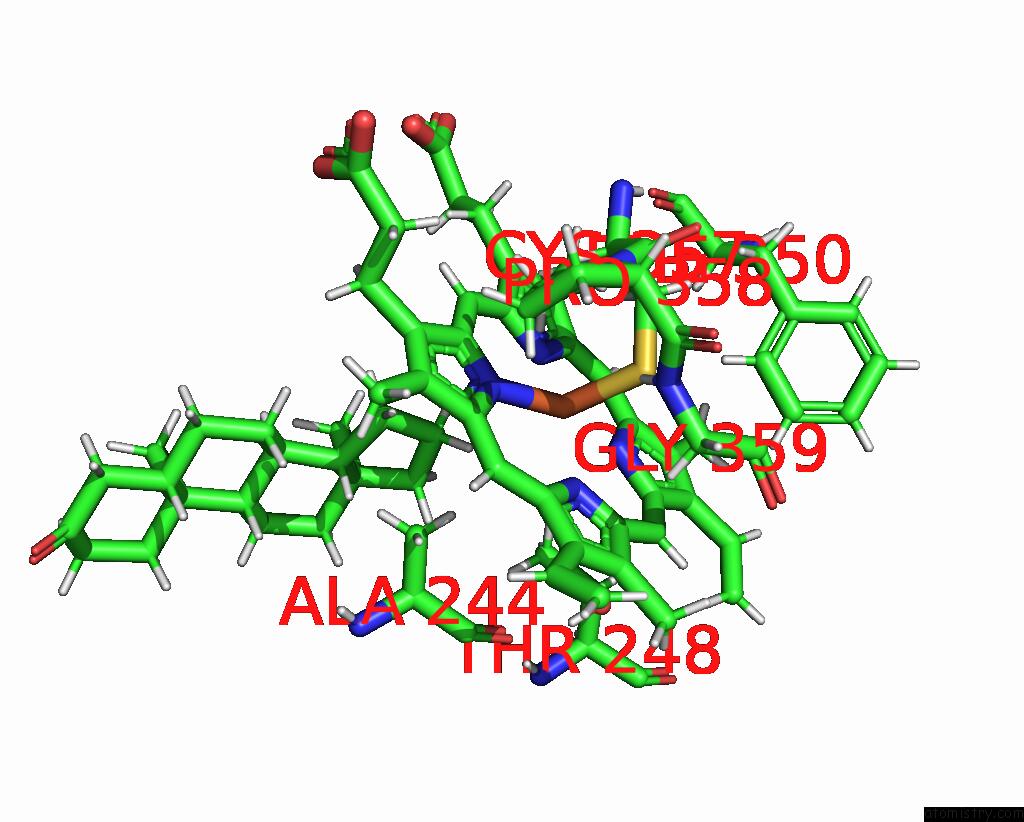
Mono view
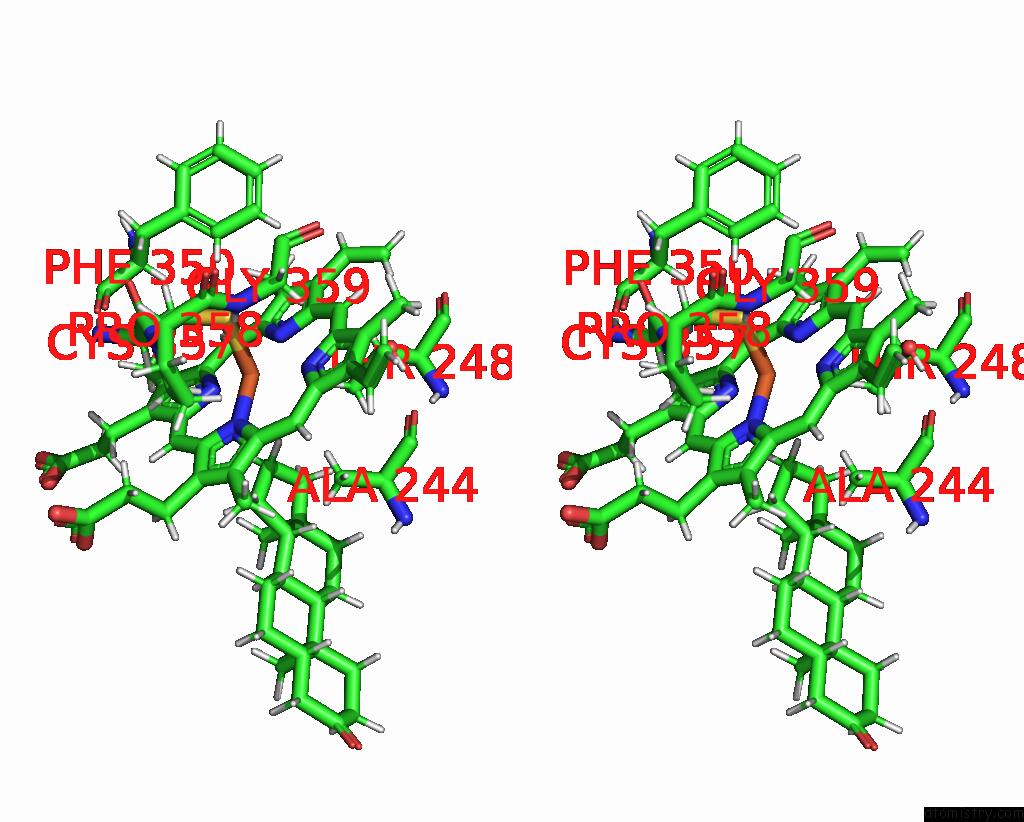
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One within 5.0Å range:
|
Iron binding site 2 out of 2 in 6to2
Go back to
Iron binding site 2 out
of 2 in the Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One
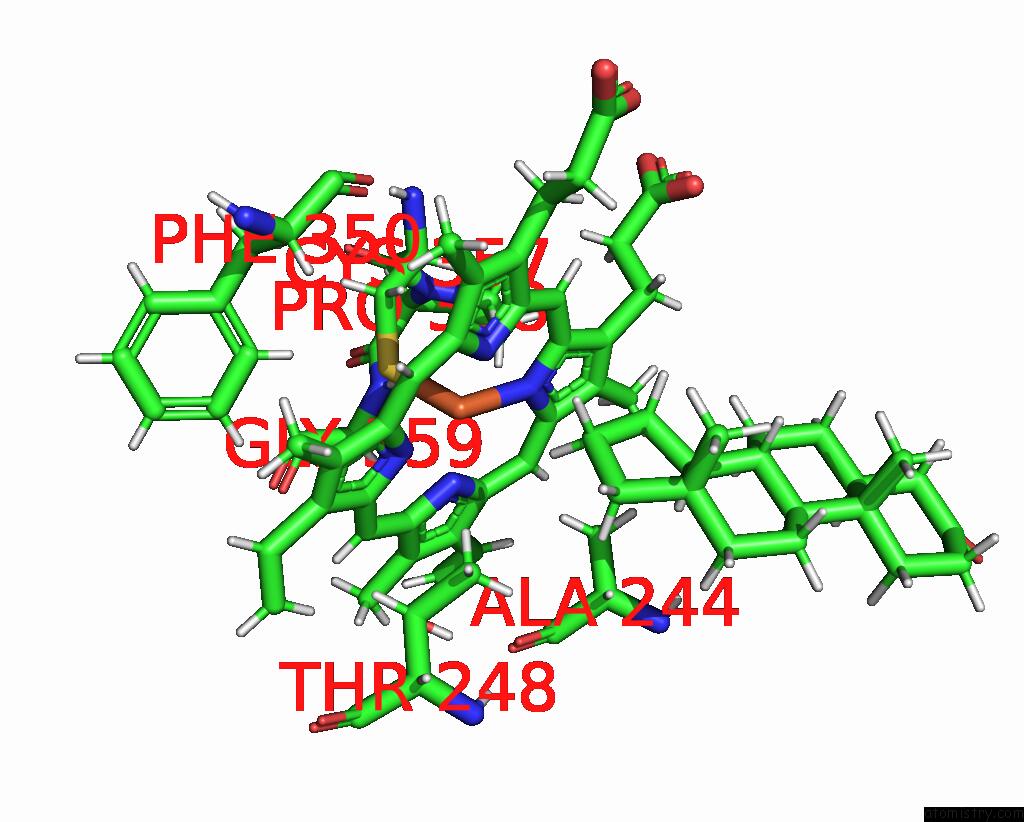
Mono view
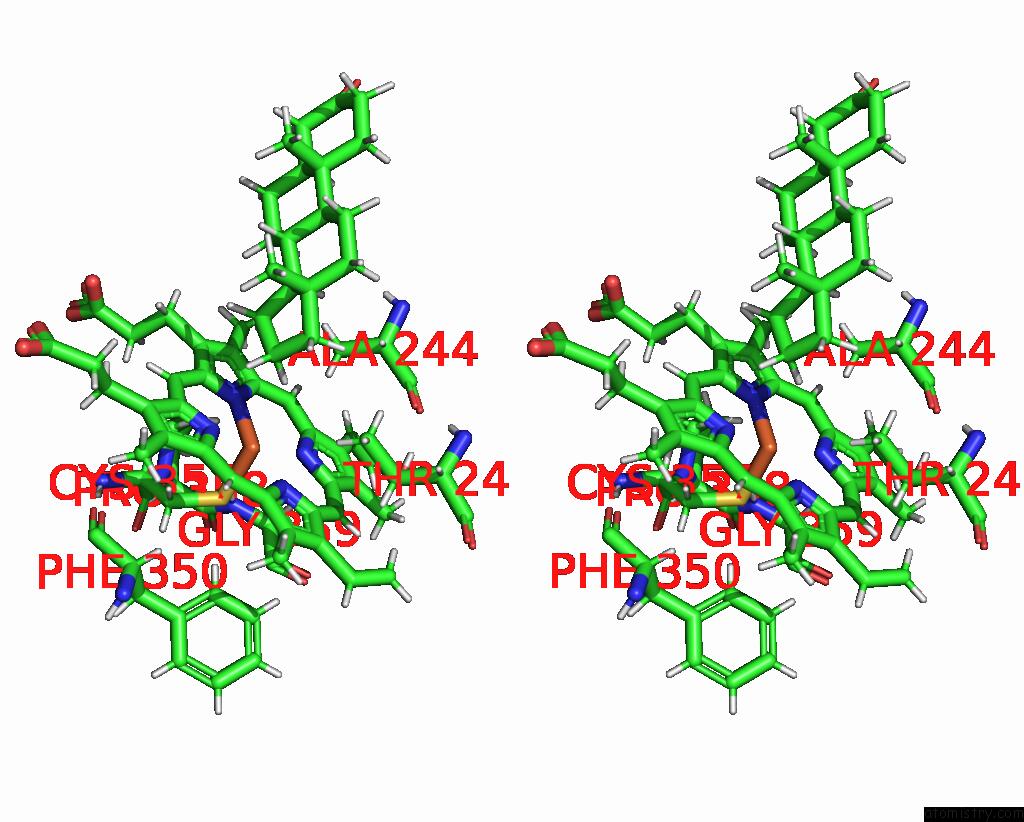
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of CYP154C5 From Nocardia Farcinica in Complex with 5ALPHA-Androstan-3-One within 5.0Å range:
|
Reference:
P.Bracco,
H.J.Wijma,
B.Nicolai,
J.A.Rodriguez Buitrago,
T.Klunemann,
A.Vila,
P.Schrepfer,
W.Blankenfeldt,
D.B.Janssen,
A.Schallmey.
CYP154C5 Regioselectivity in Steroid Hydroxylation Explored By Substrate Modifications and Protein Engineering. Chembiochem 2020.
ISSN: ESSN 1439-7633
PubMed: 33145893
DOI: 10.1002/CBIC.202000735
Page generated: Wed Aug 6 14:13:04 2025
ISSN: ESSN 1439-7633
PubMed: 33145893
DOI: 10.1002/CBIC.202000735
Last articles
K in 7PQTK in 7PS8
K in 7PV8
K in 7POZ
K in 7PNL
K in 7POE
K in 7PLK
K in 7PKA
K in 7PHK
K in 7PHL