Iron »
PDB 9fox-9g03 »
9fzy »
Iron in PDB 9fzy: Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
Enzymatic activity of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
All present enzymatic activity of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A):
1.2.7.4; 2.3.1.169;
1.2.7.4; 2.3.1.169;
Other elements in 9fzy:
The structure of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) also contains other interesting chemical elements:
| Cobalt | (Co) | 1 atom |
| Nickel | (Ni) | 4 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 28;Binding sites:
The binding sites of Iron atom in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) (pdb code 9fzy). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 28 binding sites of Iron where determined in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A), PDB code: 9fzy:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 28 in 9fzy
Go back to
Iron binding site 1 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
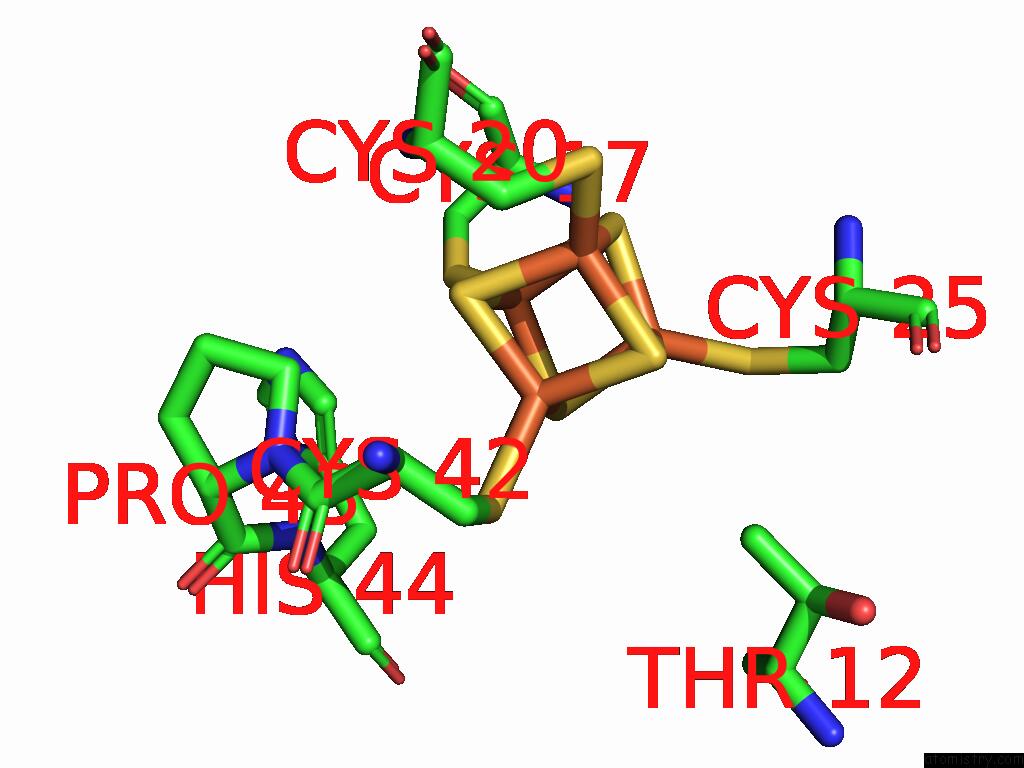
Mono view
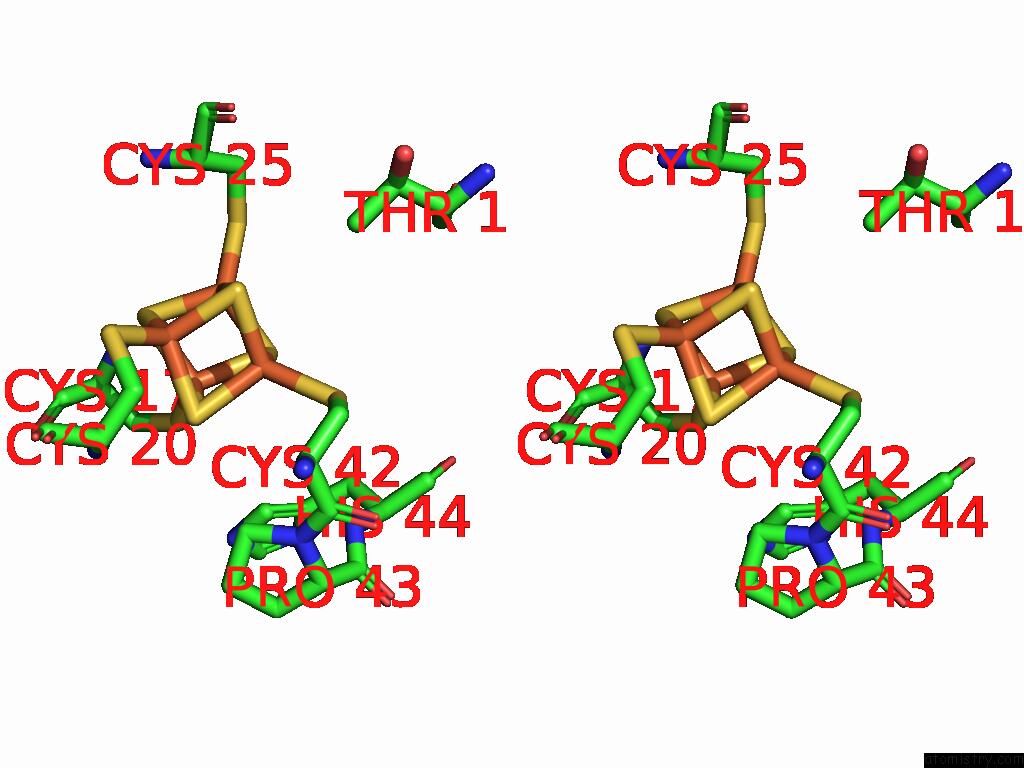
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Iron binding site 2 out of 28 in 9fzy
Go back to
Iron binding site 2 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
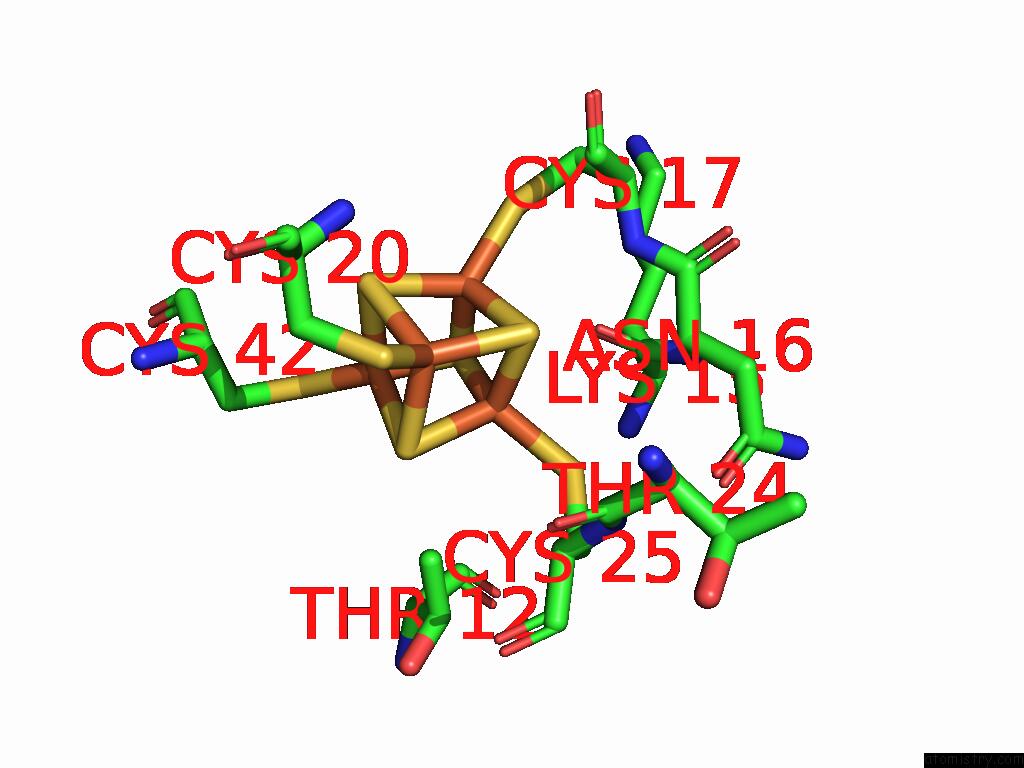
Mono view
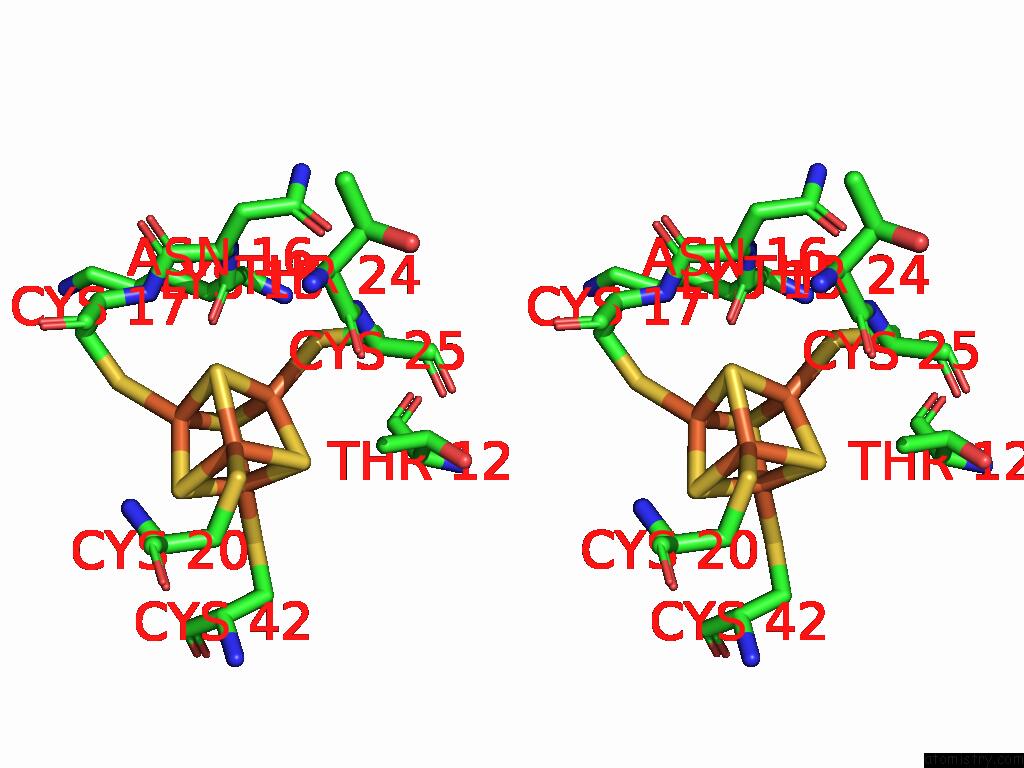
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
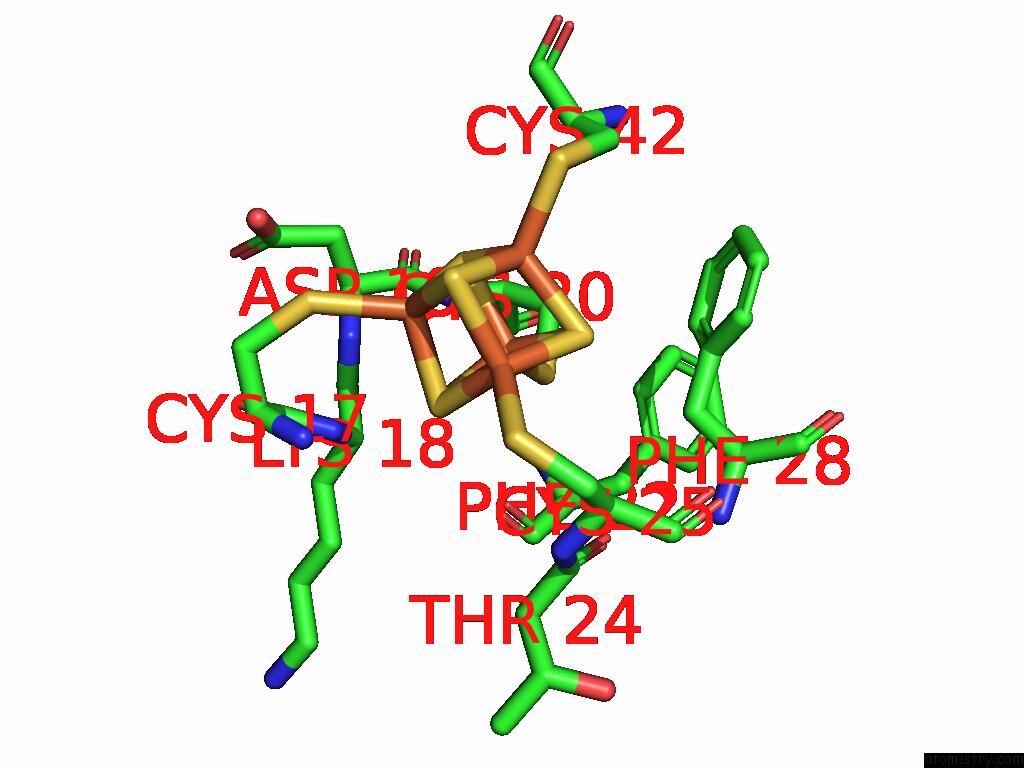
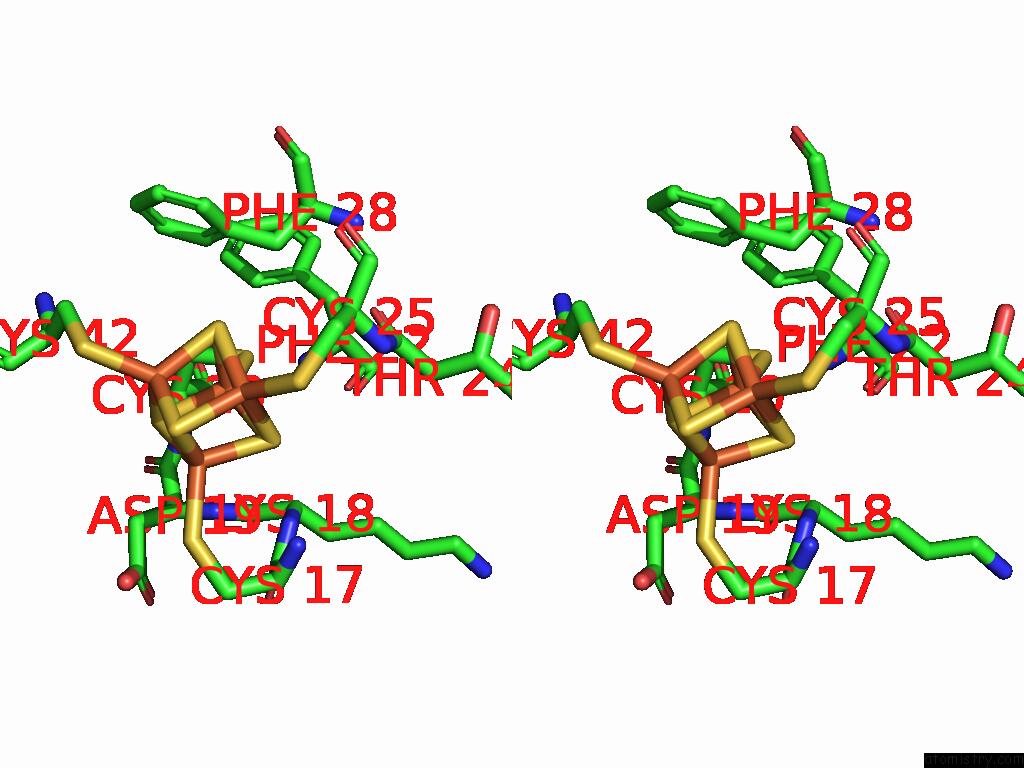
Iron binding site 3 out of 28 in 9fzy
Go back to
Iron binding site 3 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
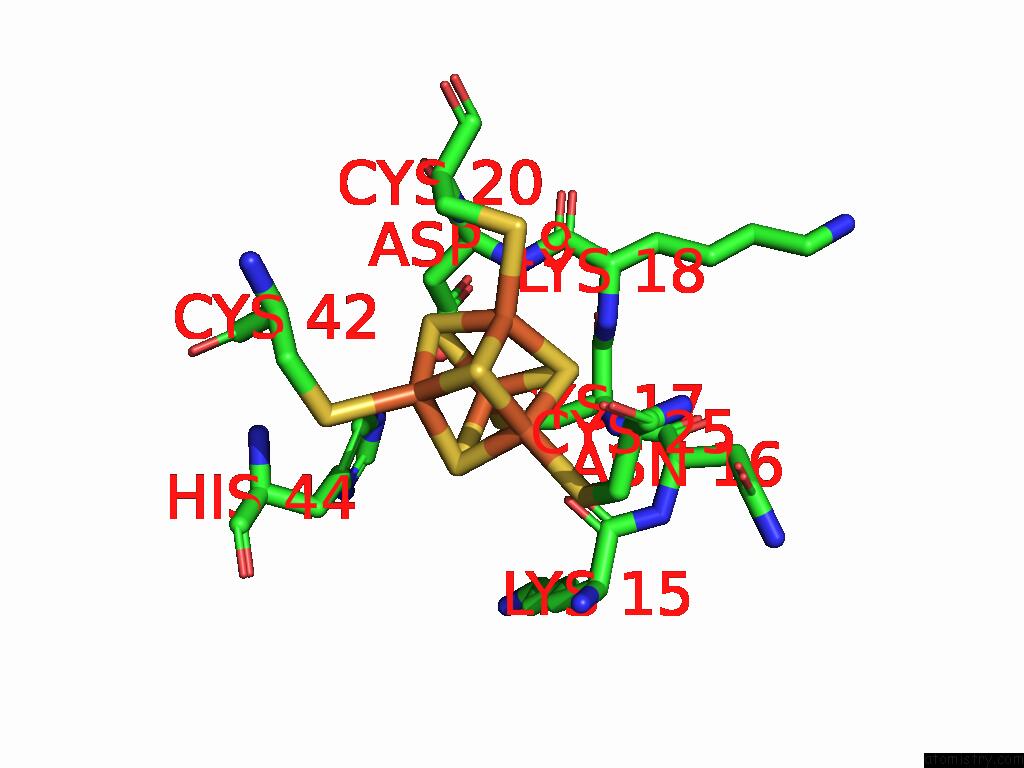
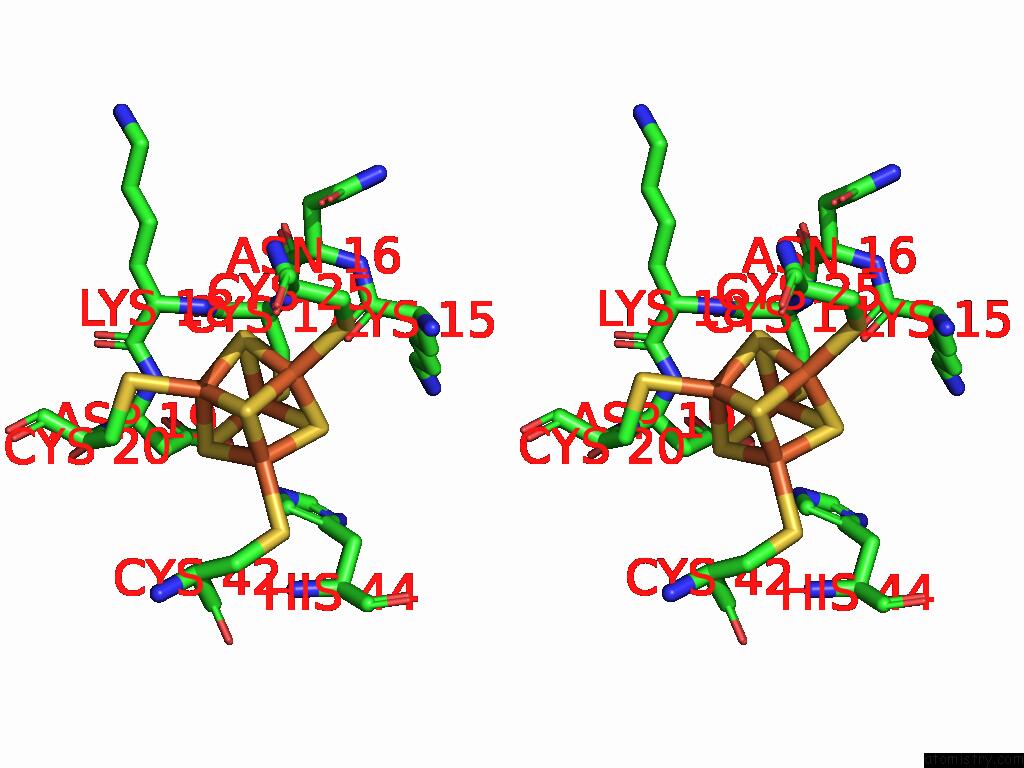
Iron binding site 4 out of 28 in 9fzy
Go back to
Iron binding site 4 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
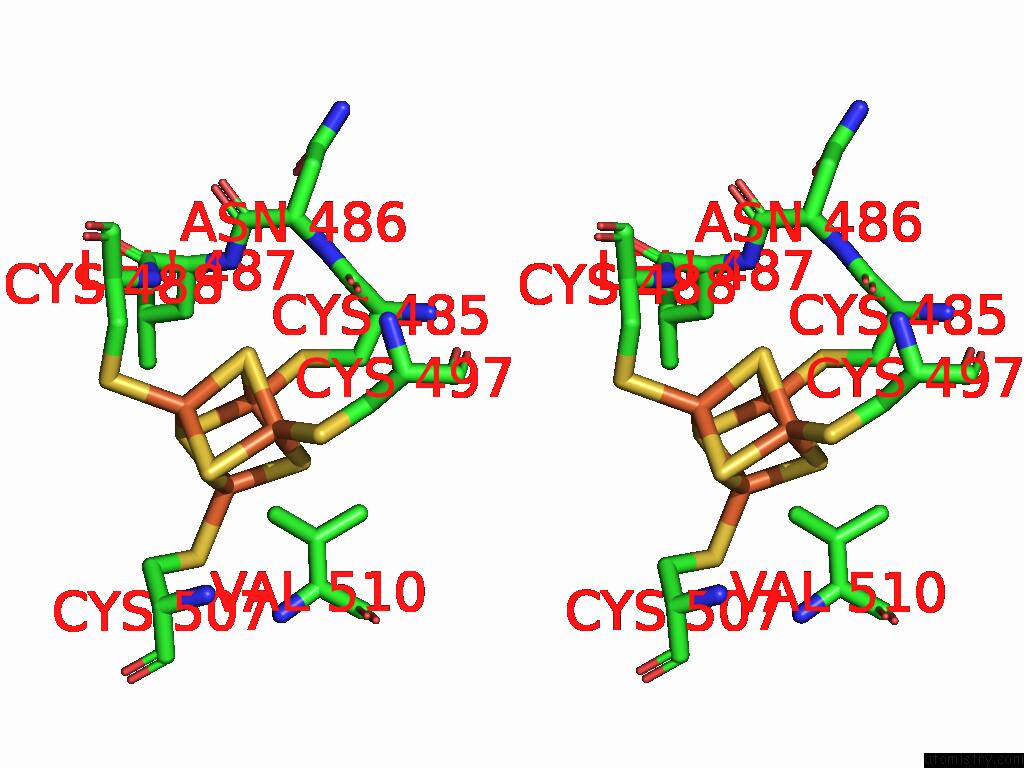
Iron binding site 5 out of 28 in 9fzy
Go back to
Iron binding site 5 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
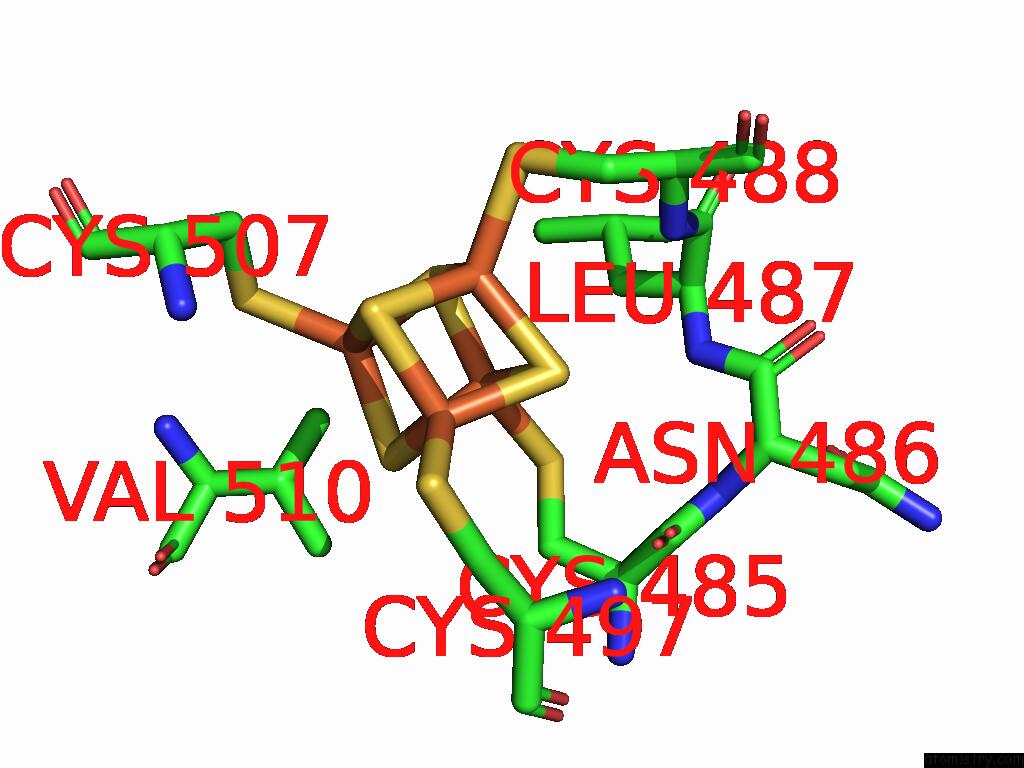
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
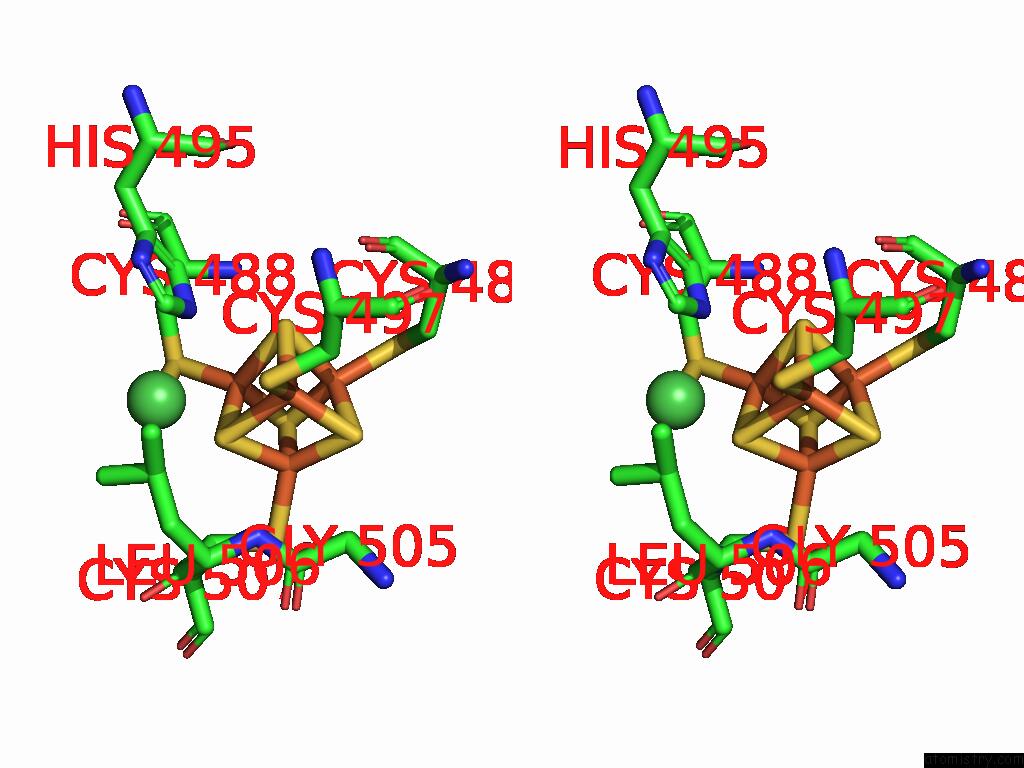
Iron binding site 6 out of 28 in 9fzy
Go back to
Iron binding site 6 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
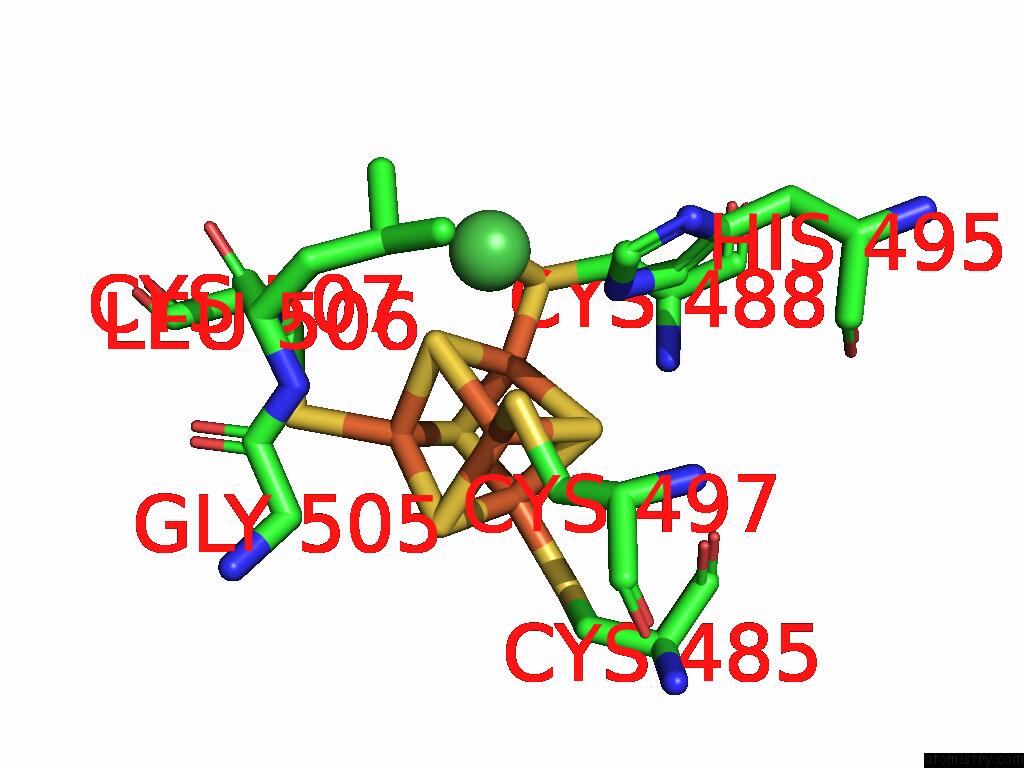
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Iron binding site 7 out of 28 in 9fzy
Go back to
Iron binding site 7 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
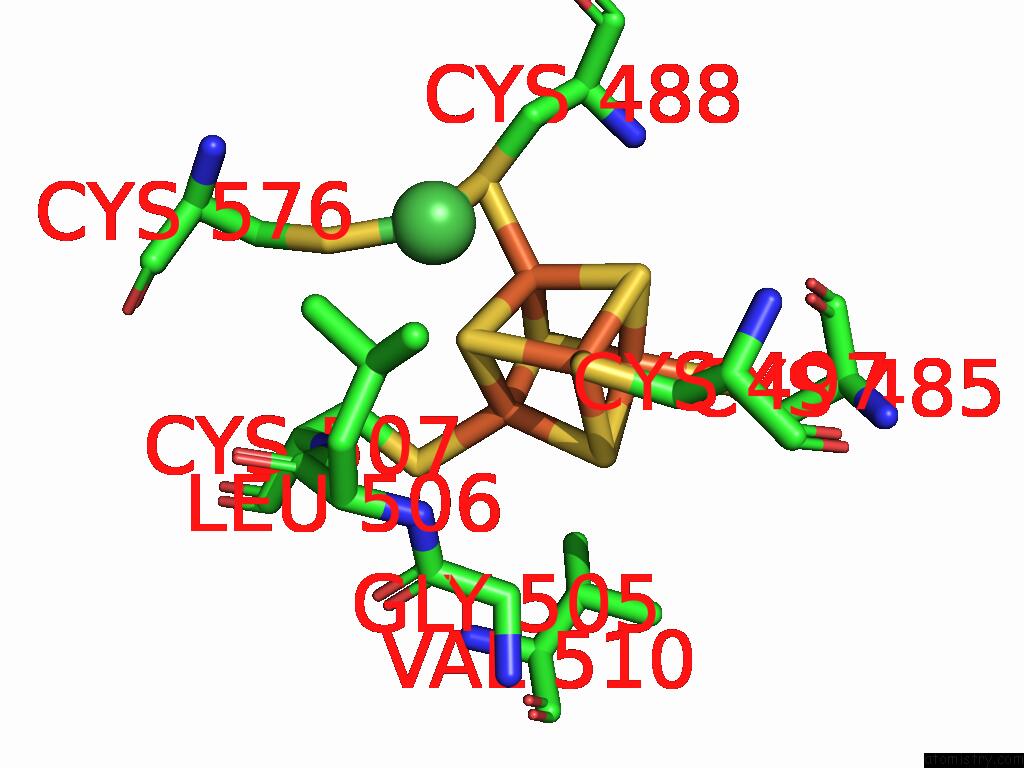
Mono view
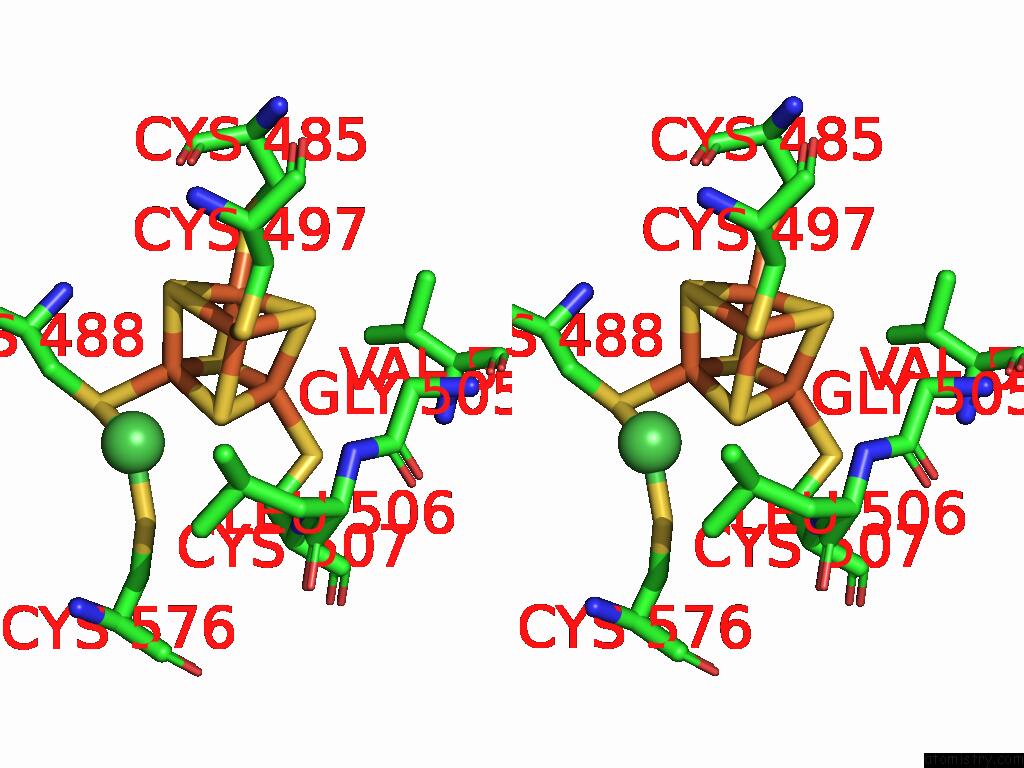
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Iron binding site 8 out of 28 in 9fzy
Go back to
Iron binding site 8 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
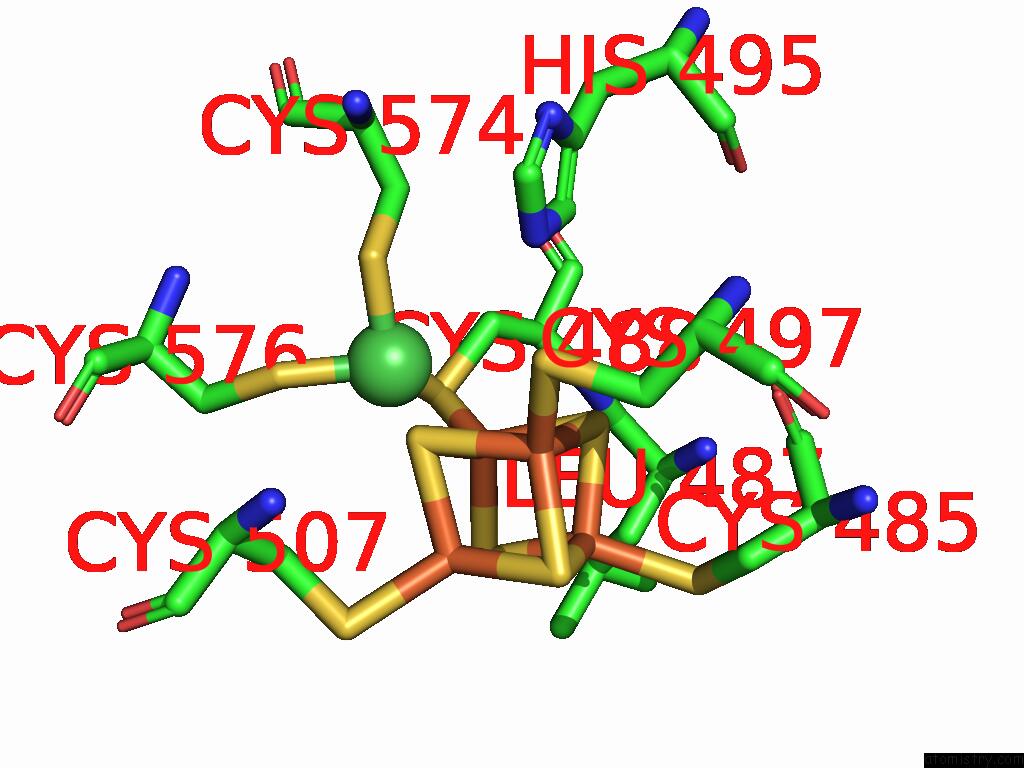
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Iron binding site 9 out of 28 in 9fzy
Go back to
Iron binding site 9 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
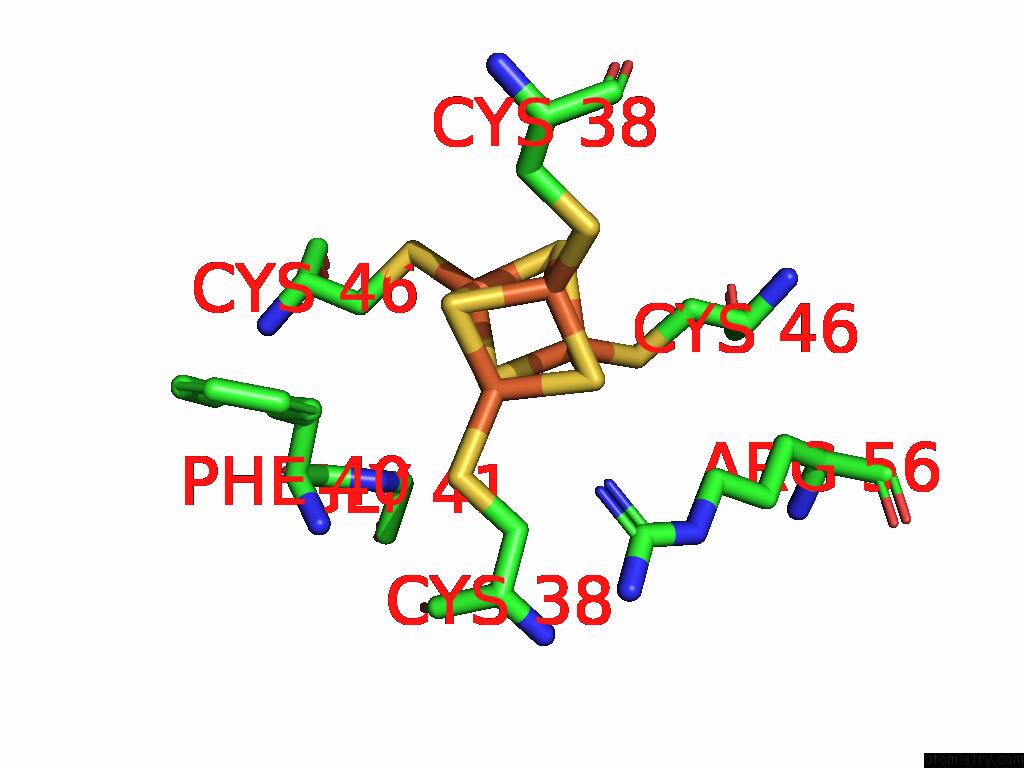
Mono view
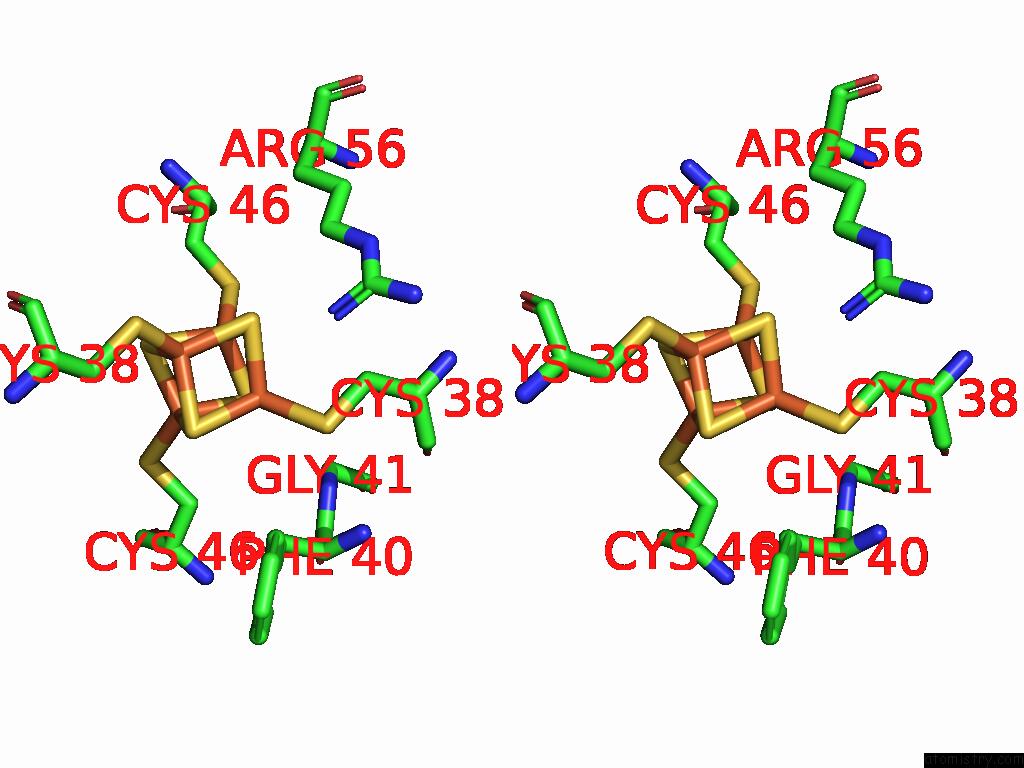
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Iron binding site 10 out of 28 in 9fzy
Go back to
Iron binding site 10 out
of 28 in the Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A)
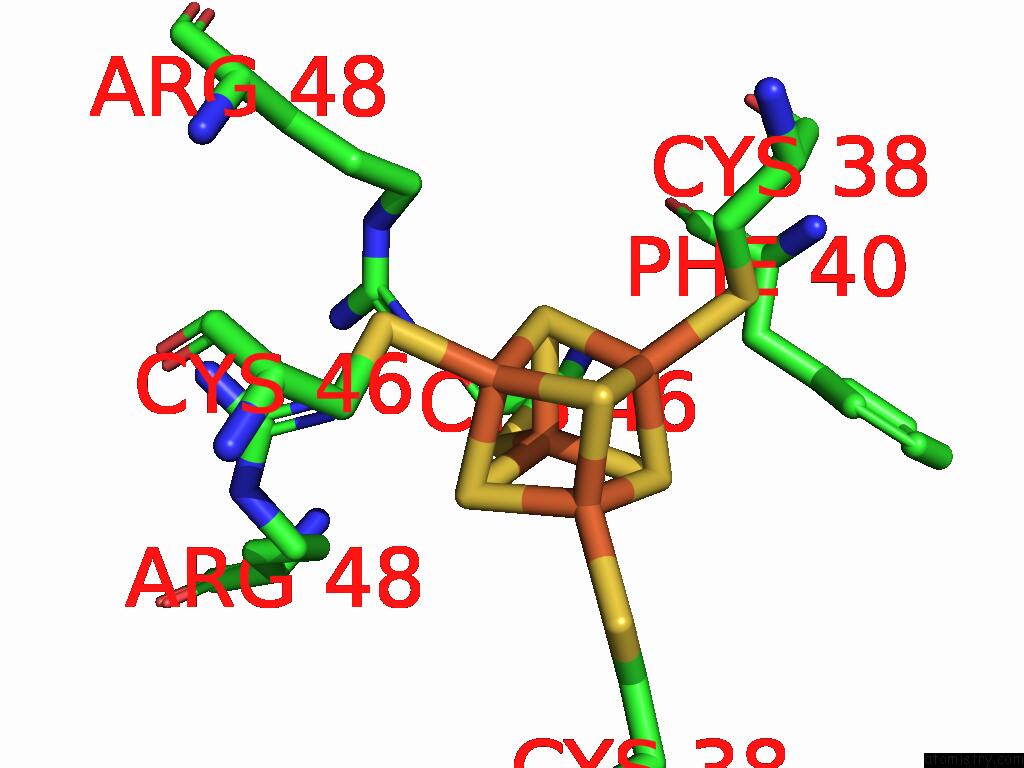
Mono view
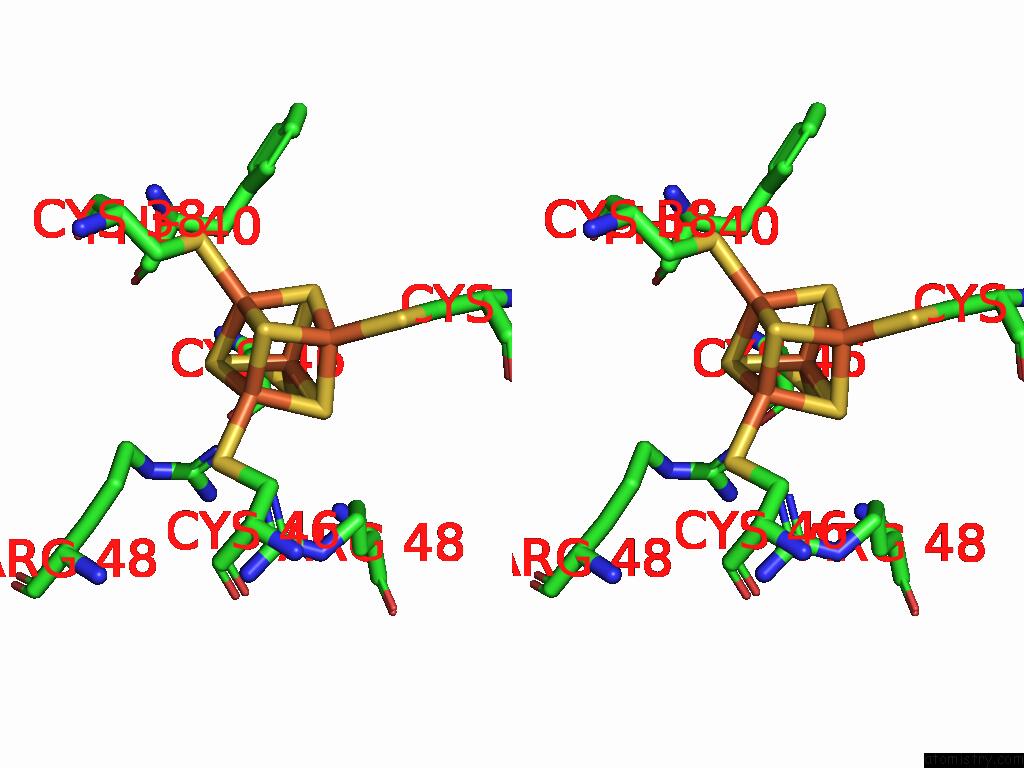
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Structure of Carbon Monoxide Dehydrogenase/Acetyl-Coa Synthase (Codh/Acs) in Complex with Corrinoid Iron-Sulfur Protein (Cofesp) From Clostridium Autoethanogenum (Composite Structure, Class 3A) within 5.0Å range:
|
Reference:
M.D.Yin,
O.N.Lemaire,
M.Belhamri,
J.G.Rosas-Jimenez,
G.Hummer,
T.Wagner,
B.J.Murphy.
Snapshots of Acetyl-Coa Synthesis, the Last Step of CO2 Fixation in the Wood-Ljungdahl Pathway Science 2025.
ISSN: ESSN 1095-9203
DOI: 10.1126/SCIENCE.ADR9672
Page generated: Fri Aug 8 06:06:12 2025
ISSN: ESSN 1095-9203
DOI: 10.1126/SCIENCE.ADR9672
Last articles
Zn in 9QM9Zn in 9S44
Zn in 9OFE
Zn in 9OFC
Zn in 9OFD
Zn in 9OF1
Zn in 9OFB
Zn in 9N0J
Zn in 9M5X
Zn in 9LGI