Iron »
PDB 9fuh-9gyz »
9gmc »
Iron in PDB 9gmc: Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
Protein crystallography data
The structure of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla, PDB code: 9gmc
was solved by
E.De La Mora,
J.Ruel,
A.Usclat,
L.Martin,
P.Amara,
B.Morinaka,
Y.Nicolet,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 100.51 / 1.77 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 151.692, 62.467, 113.138, 90, 117.33, 90 |
| R / Rfree (%) | 17.8 / 21.4 |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 16;Binding sites:
The binding sites of Iron atom in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla (pdb code 9gmc). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 16 binding sites of Iron where determined in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla, PDB code: 9gmc:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
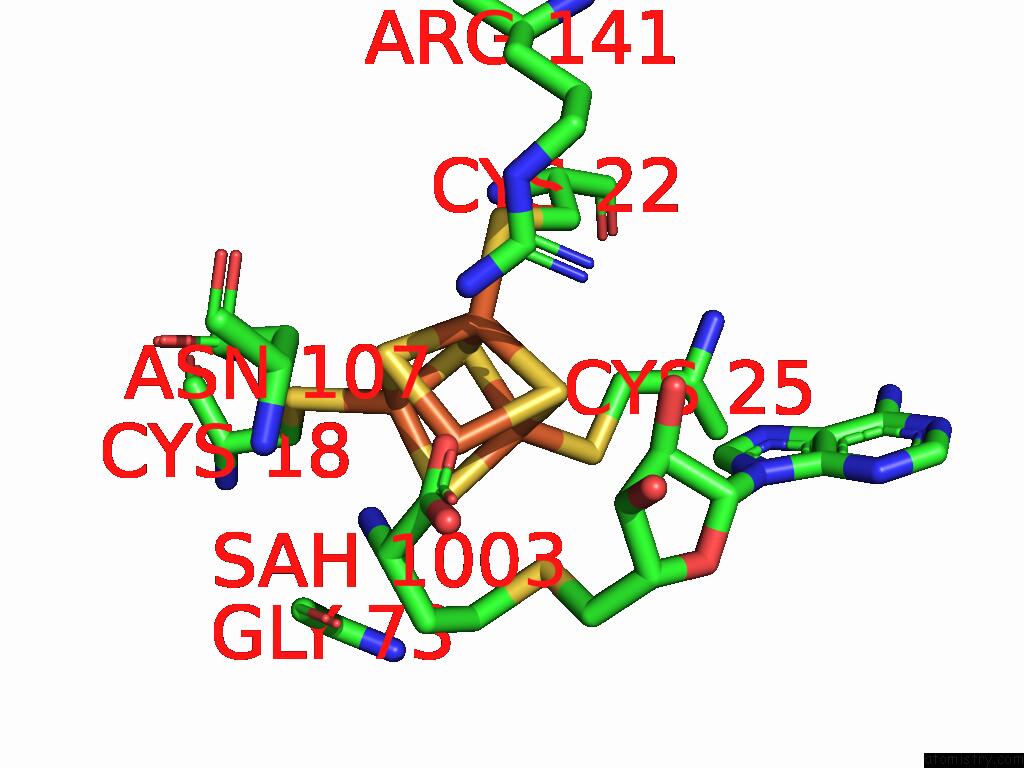
Iron binding site 1 out of 16 in 9gmc
Go back to
Iron binding site 1 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
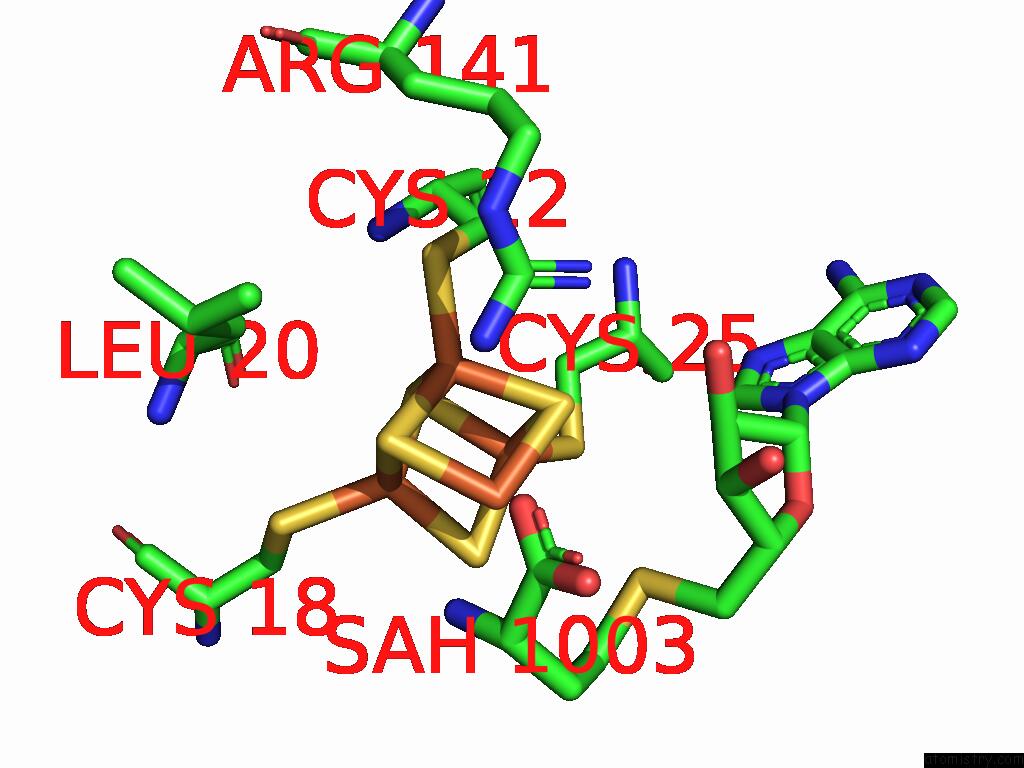
Iron binding site 2 out of 16 in 9gmc
Go back to
Iron binding site 2 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
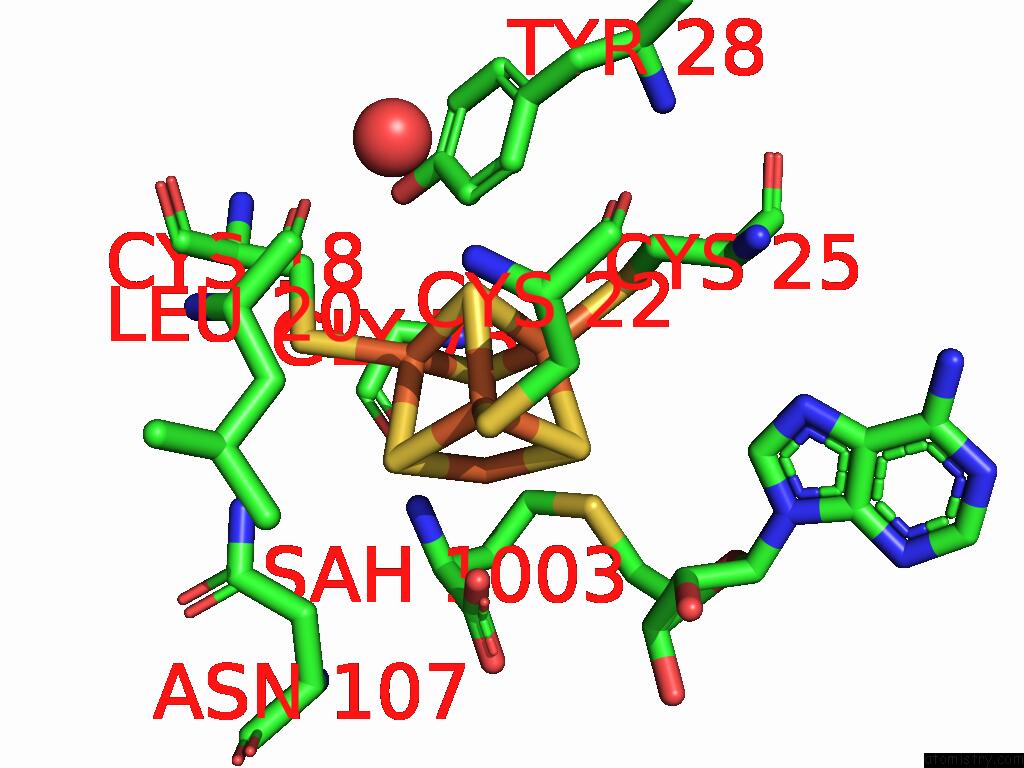
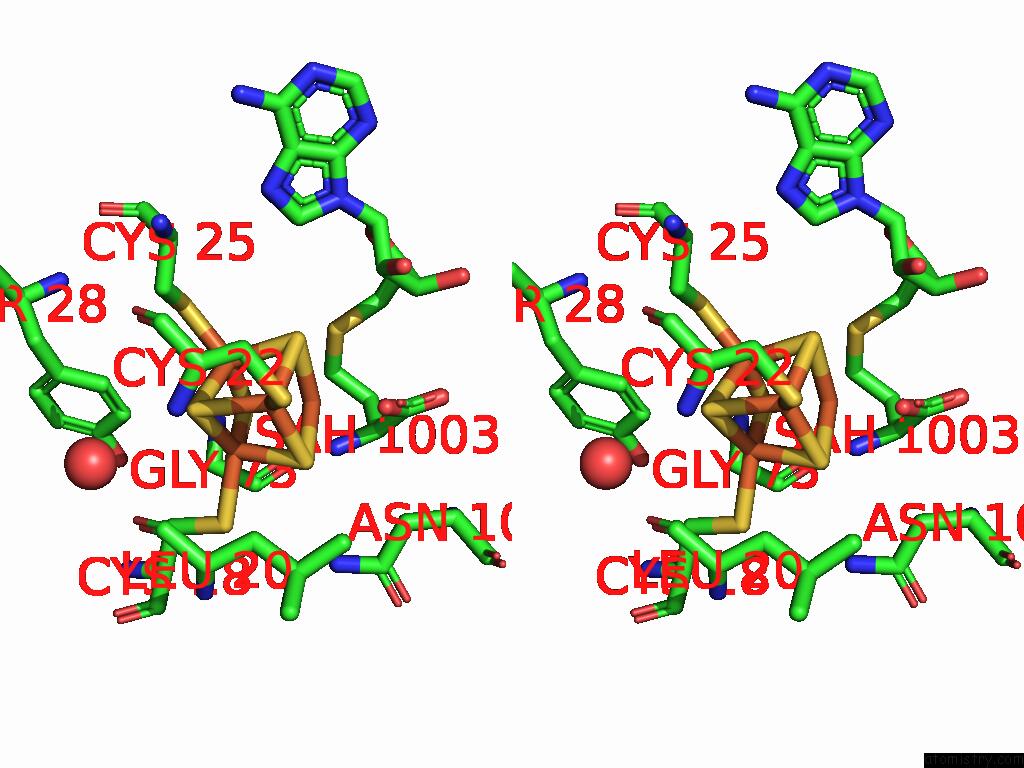
Iron binding site 3 out of 16 in 9gmc
Go back to
Iron binding site 3 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
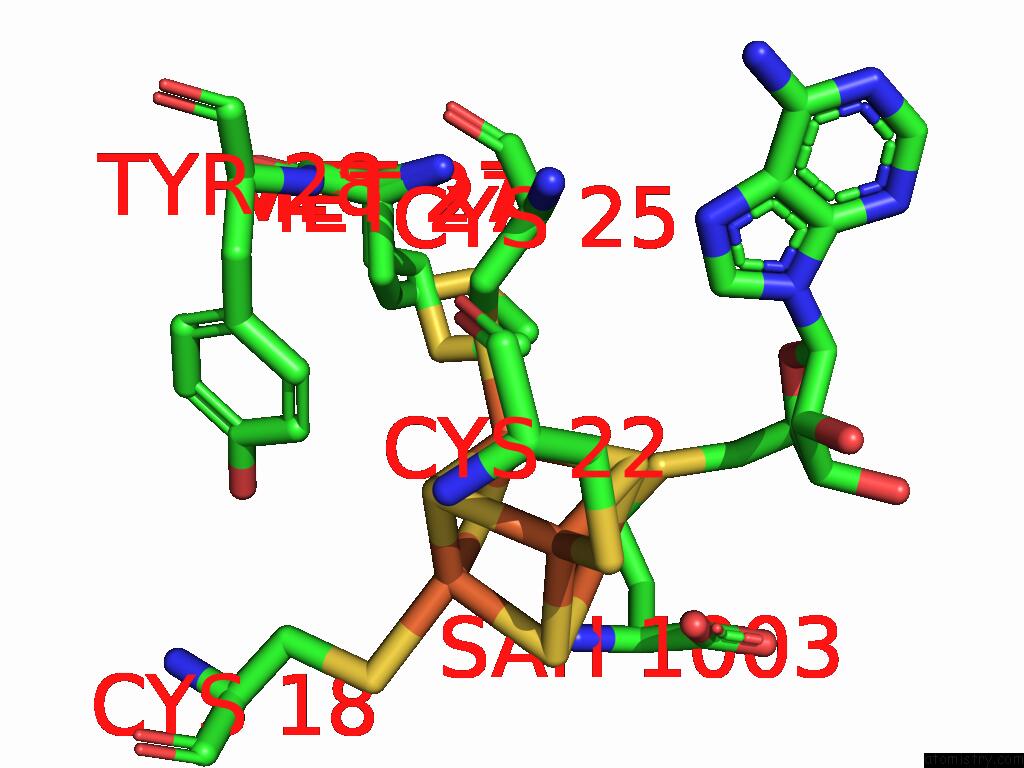
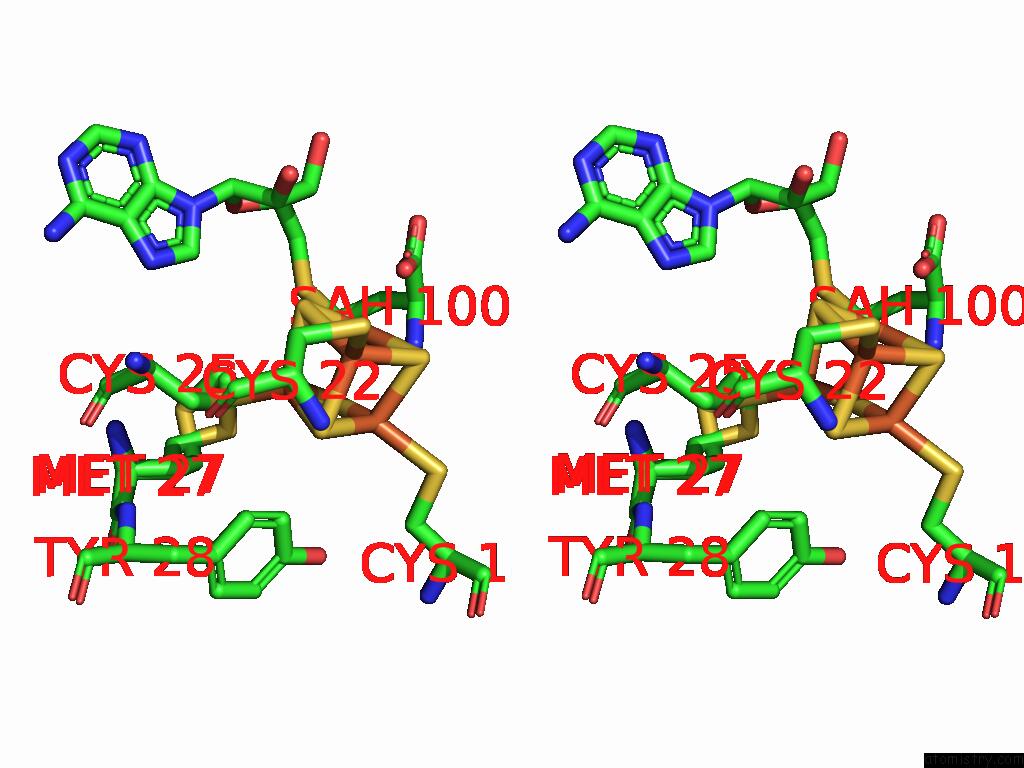
Iron binding site 4 out of 16 in 9gmc
Go back to
Iron binding site 4 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 5 out of 16 in 9gmc
Go back to
Iron binding site 5 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
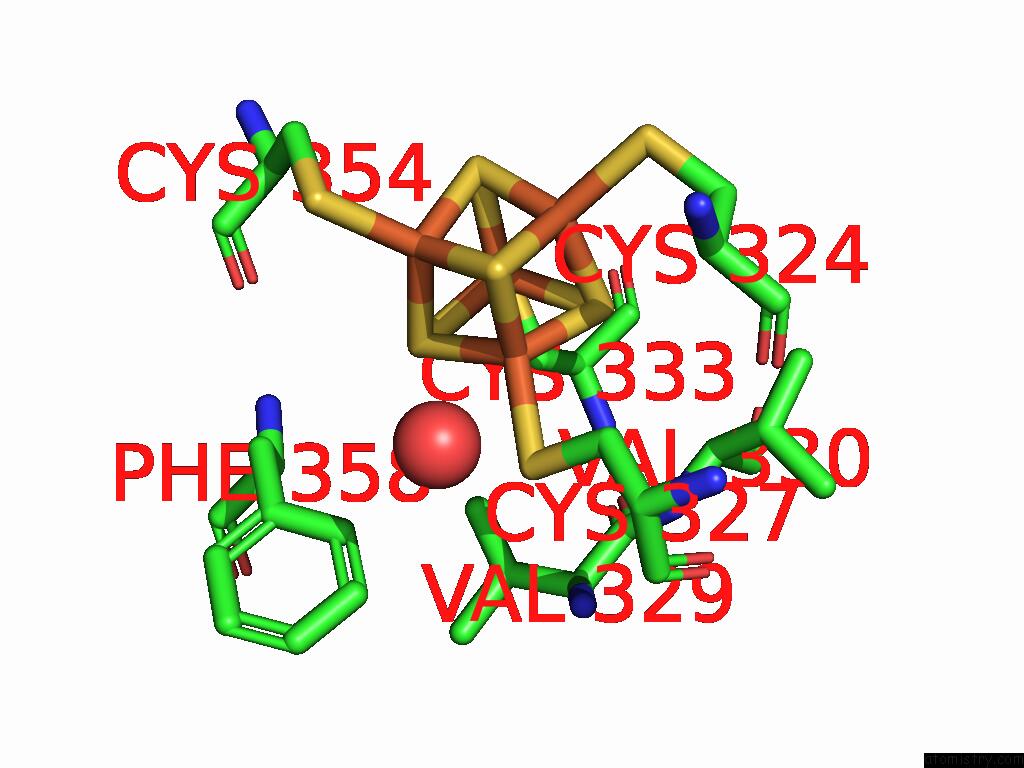
Mono view
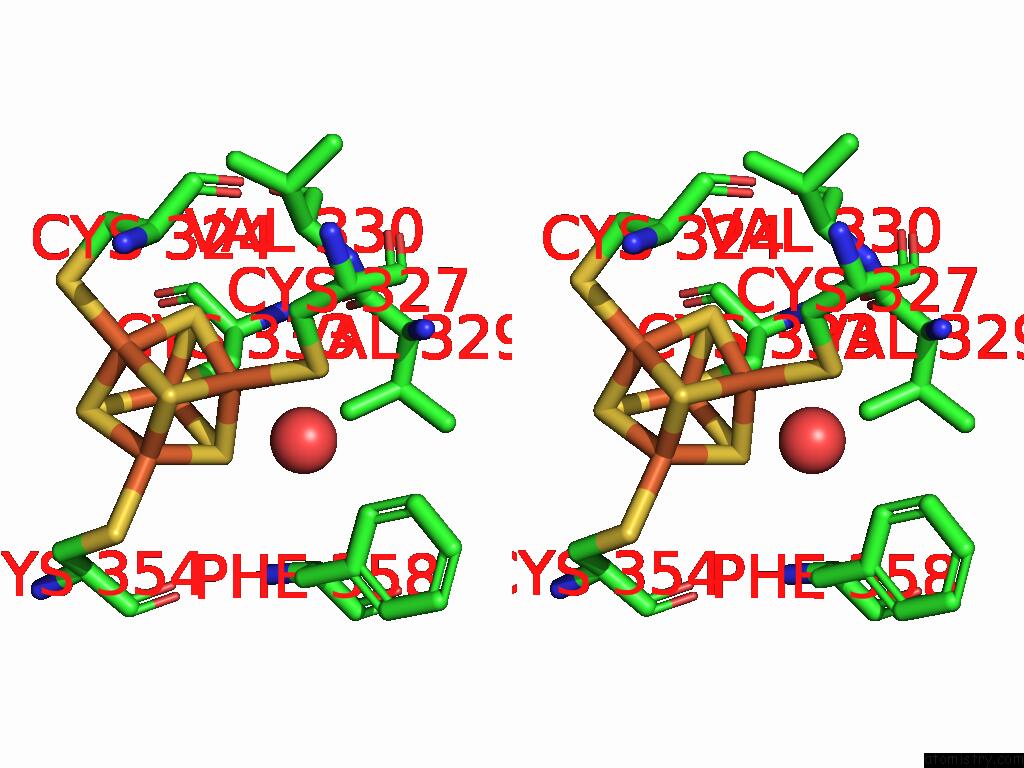
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 6 out of 16 in 9gmc
Go back to
Iron binding site 6 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
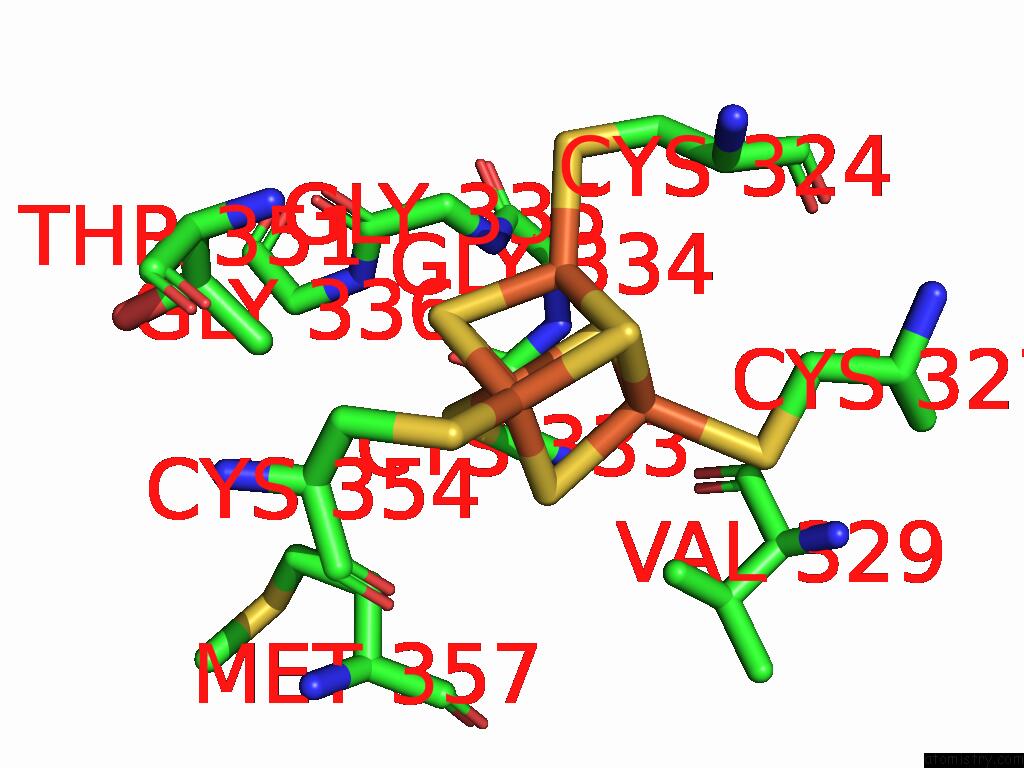
Mono view
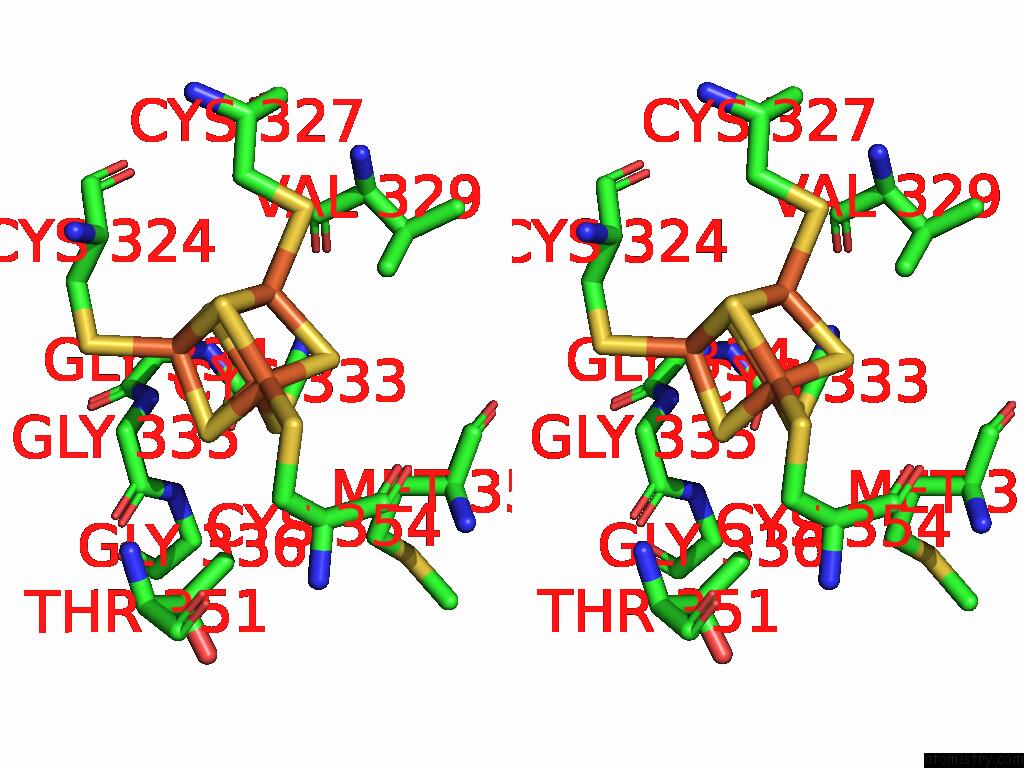
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 7 out of 16 in 9gmc
Go back to
Iron binding site 7 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
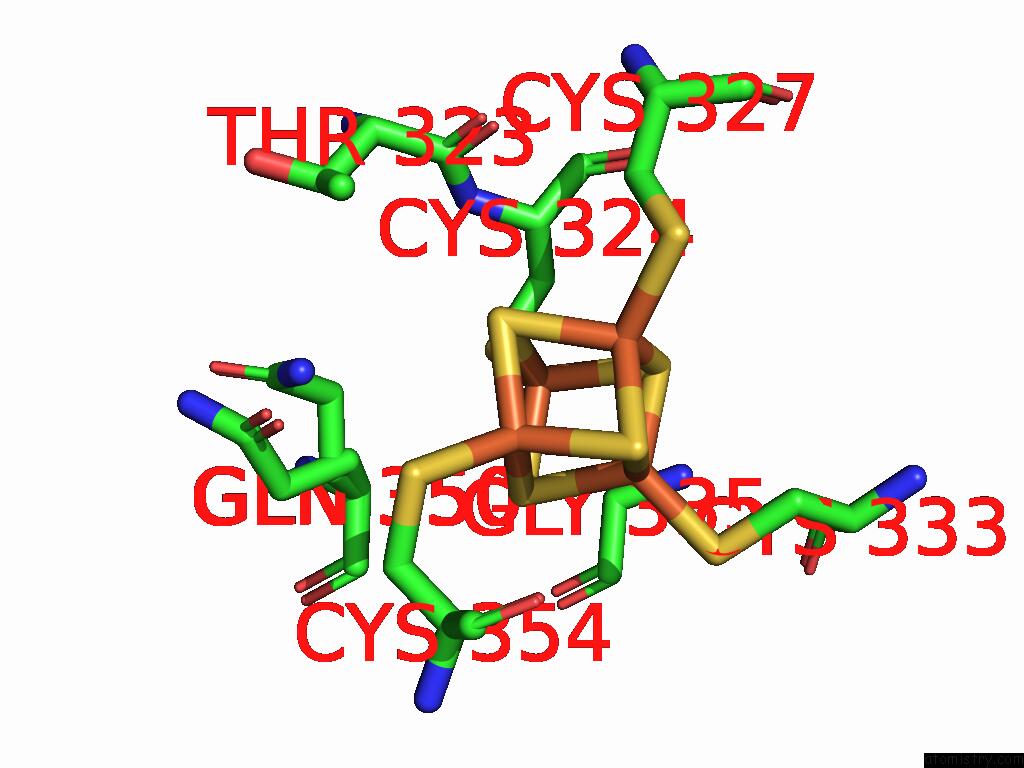
Mono view
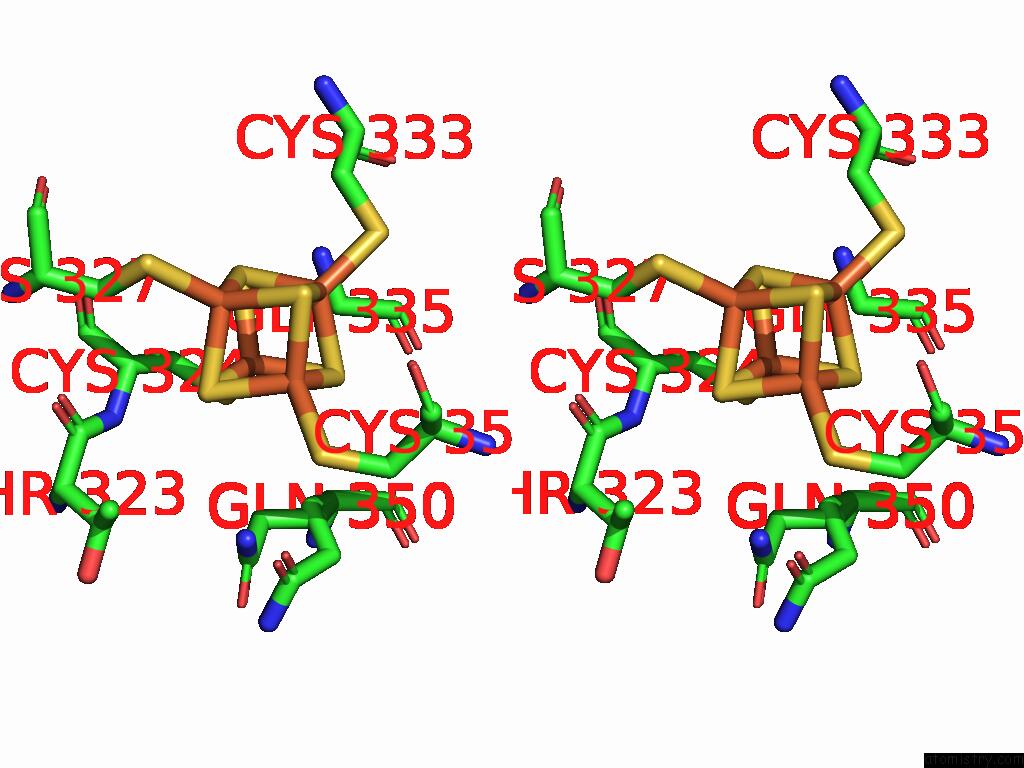
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 8 out of 16 in 9gmc
Go back to
Iron binding site 8 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
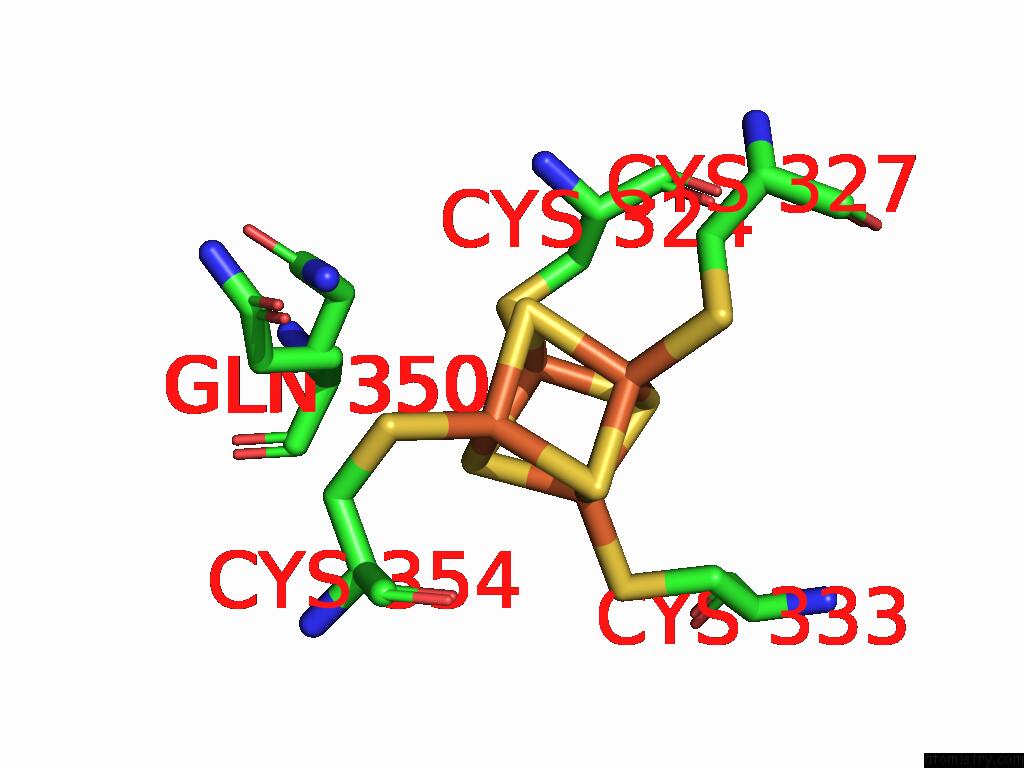
Mono view
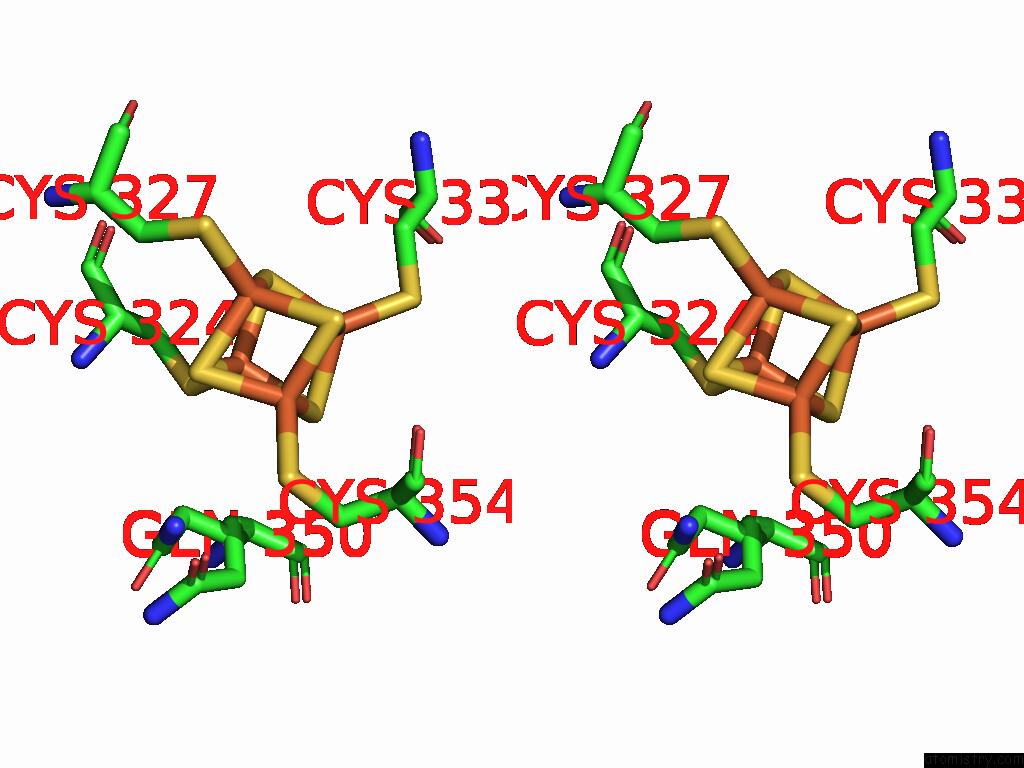
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 9 out of 16 in 9gmc
Go back to
Iron binding site 9 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
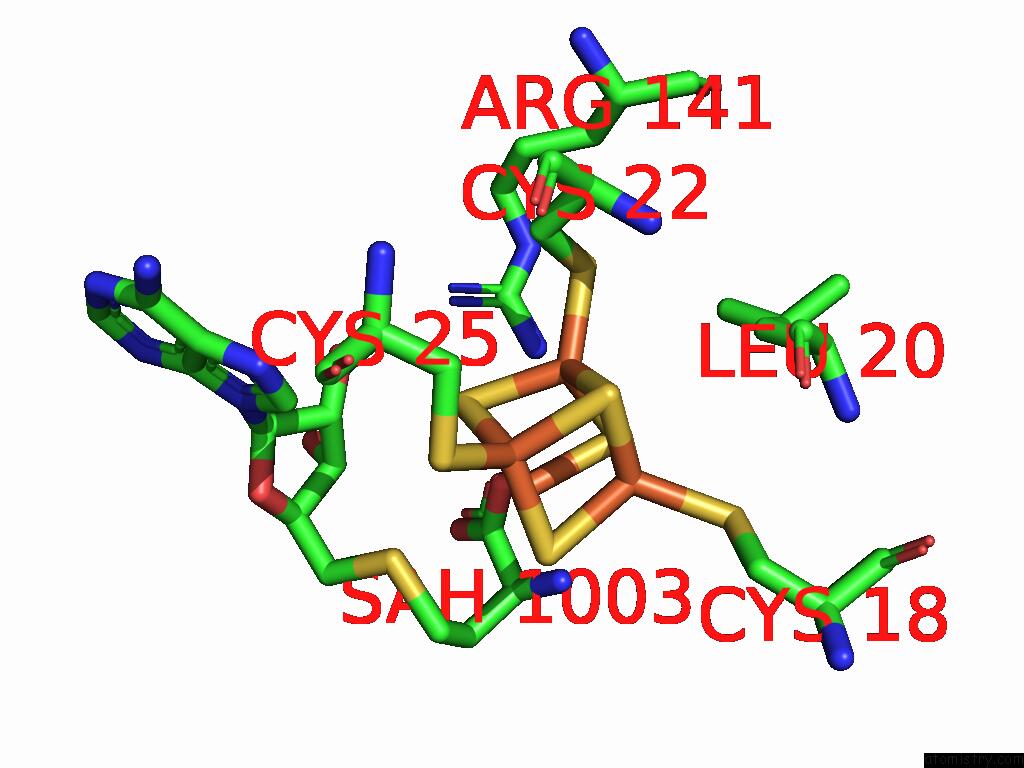
Mono view
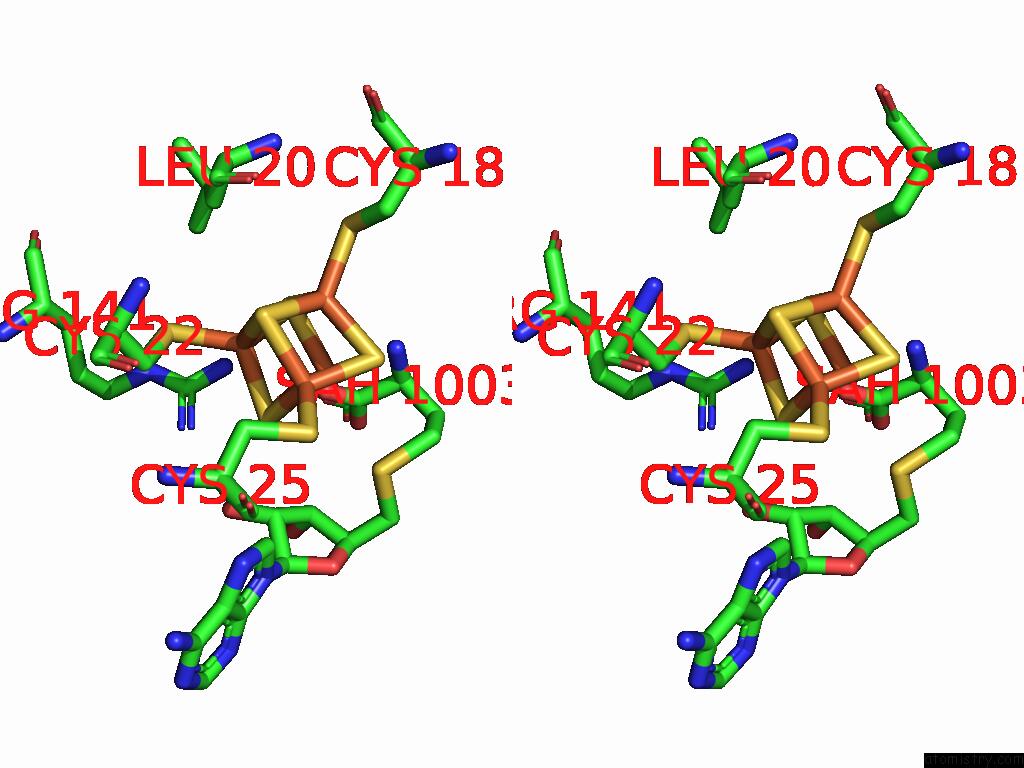
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Iron binding site 10 out of 16 in 9gmc
Go back to
Iron binding site 10 out
of 16 in the Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla
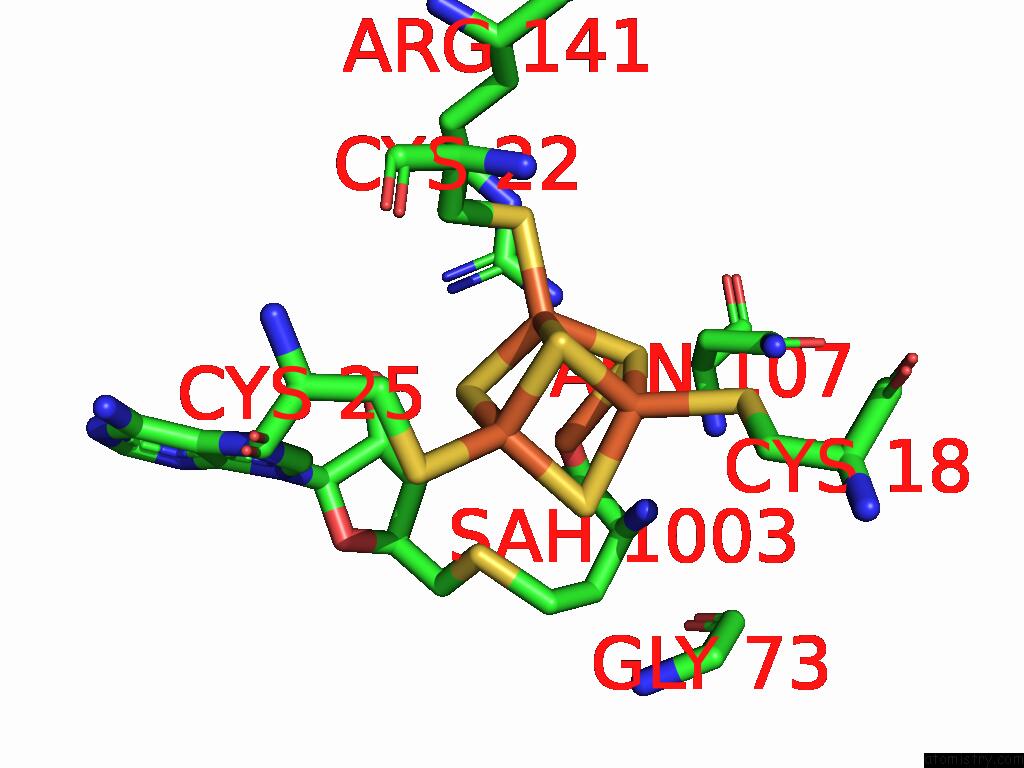
Mono view
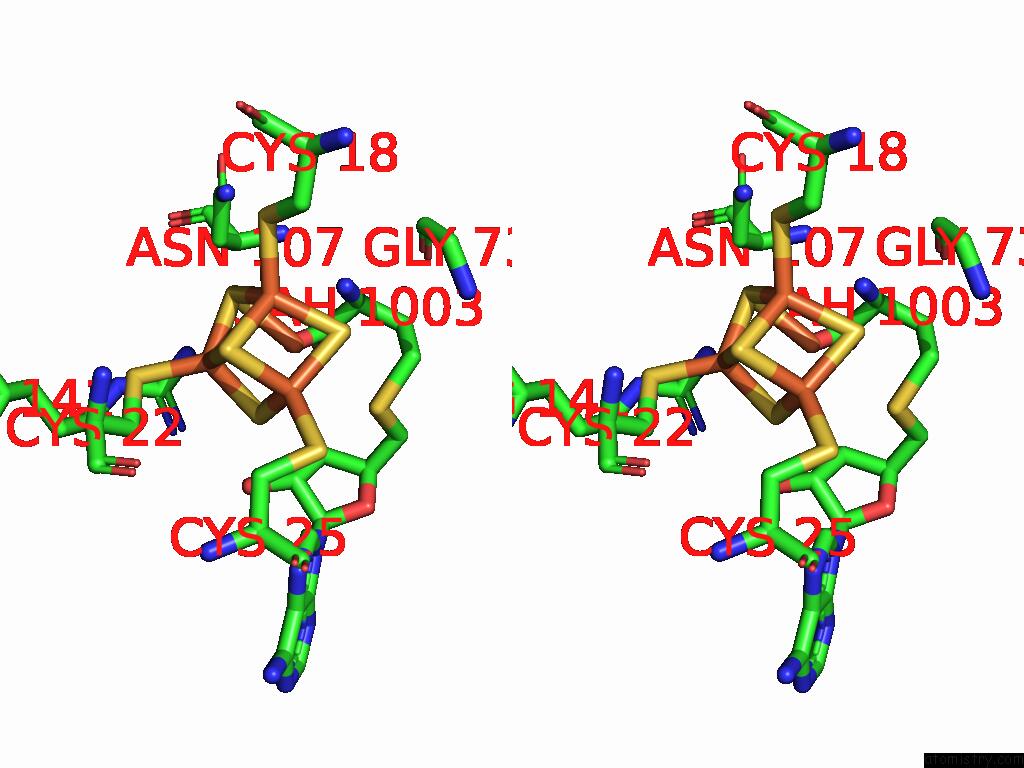
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Crystal Structure of the Complex Formed Between the Radical Sam Protein Chlb and the R3A Mutant of Chla within 5.0Å range:
|
Reference:
J.Ruel,
T.Q.N.Nguyen,
Y.Morishita,
A.Usclat,
L.Martin,
P.Amara,
S.Kieffer-Jaquinod,
M.C.Stefanoiu,
E.De La Mora,
B.I.Morinaka,
Y.Nicolet.
Peptide Recognition and Mechanism of the Radical S -Adenosyl-L-Methionine Multiple Cyclophane Synthase Chlb. J.Am.Chem.Soc. V. 147 16850 2025.
ISSN: ESSN 1520-5126
PubMed: 40354606
DOI: 10.1021/JACS.4C16004
Page generated: Fri Aug 8 06:24:37 2025
ISSN: ESSN 1520-5126
PubMed: 40354606
DOI: 10.1021/JACS.4C16004
Last articles
Hg in 1CINHg in 1CAN
Hg in 1CIM
Hg in 1CC8
Hg in 1CA3
Hg in 1BV3
Hg in 1BNW
Hg in 1BNV
Hg in 1BNU
Hg in 1BNT