Iron »
PDB 9kqb-9n5u »
9ktl »
Iron in PDB 9ktl: Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
Enzymatic activity of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
All present enzymatic activity of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh:
1.17.1.9;
1.17.1.9;
Other elements in 9ktl:
The structure of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh also contains other interesting chemical elements:
| Molybdenum | (Mo) | 2 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 30; Page 4, Binding sites: 31 - 40; Page 5, Binding sites: 41 - 48;Binding sites:
The binding sites of Iron atom in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh (pdb code 9ktl). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 48 binding sites of Iron where determined in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh, PDB code: 9ktl:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 48 in 9ktl
Go back to
Iron binding site 1 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
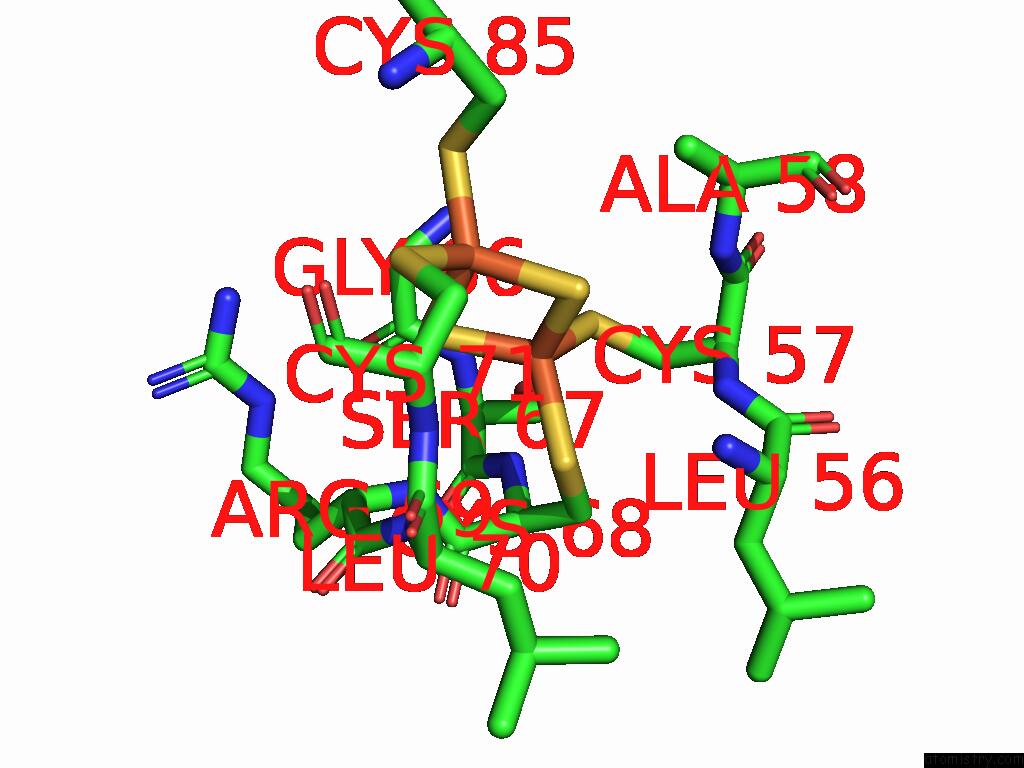
Mono view
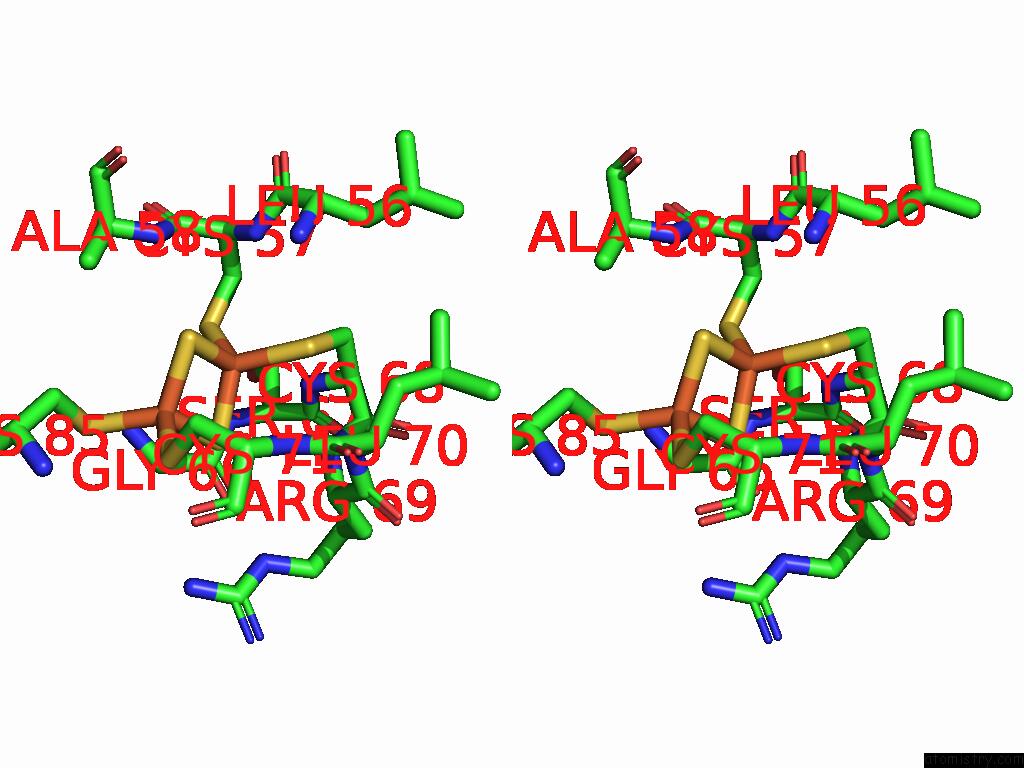
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
Iron binding site 2 out of 48 in 9ktl
Go back to
Iron binding site 2 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
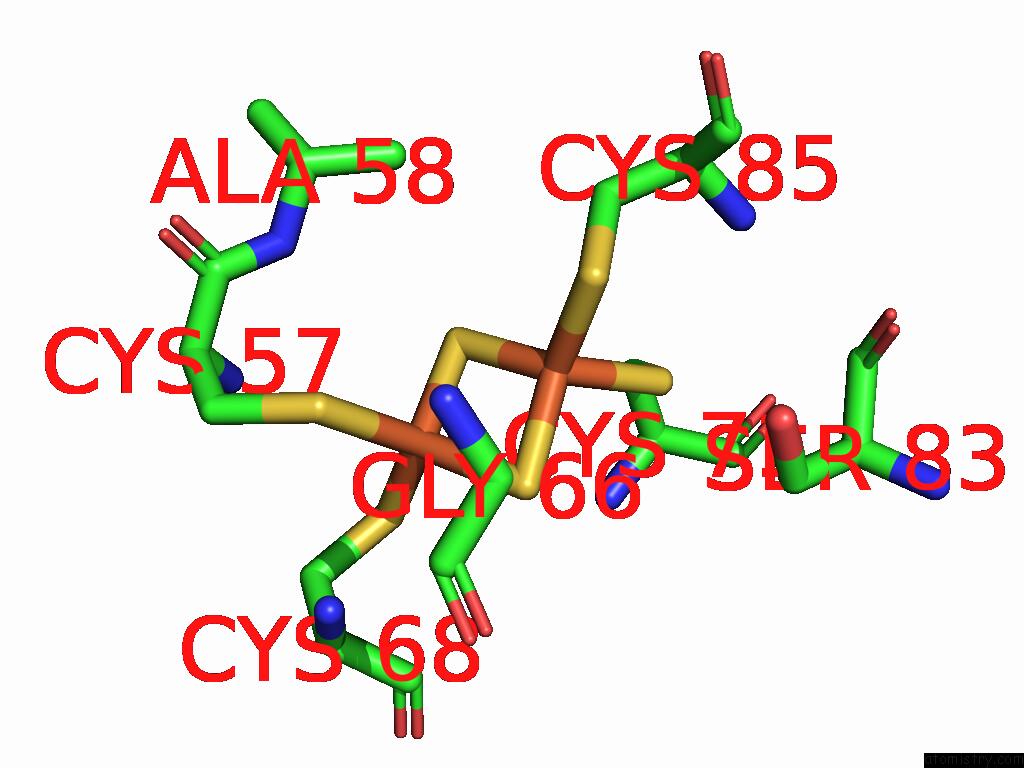
Mono view
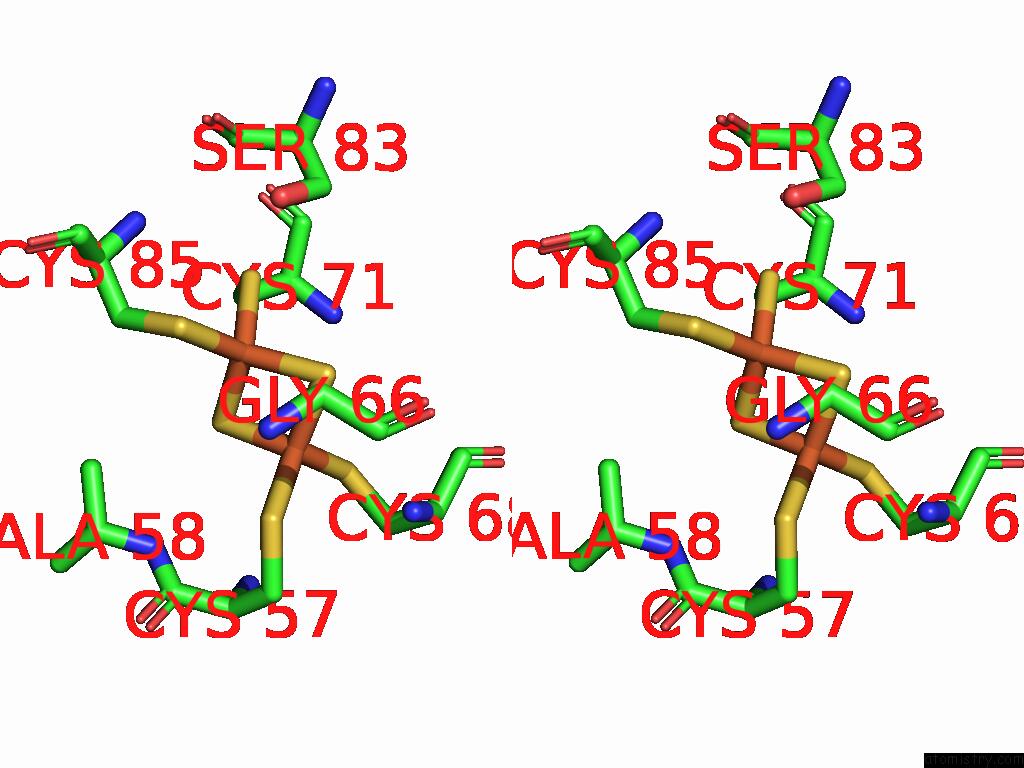
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
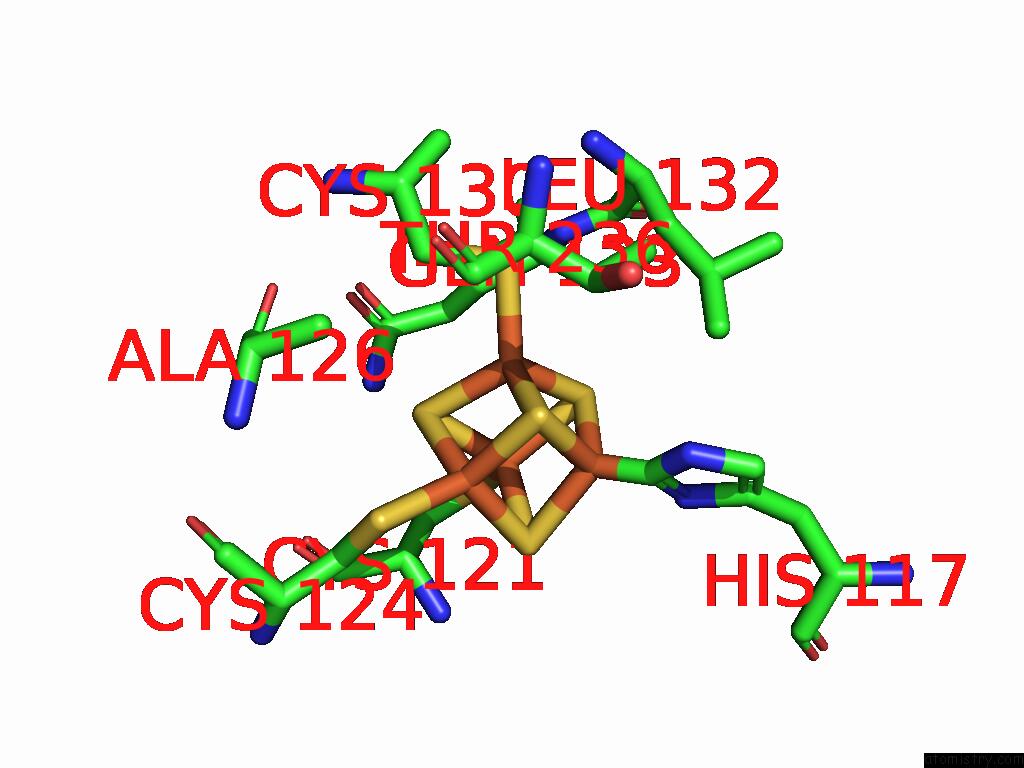
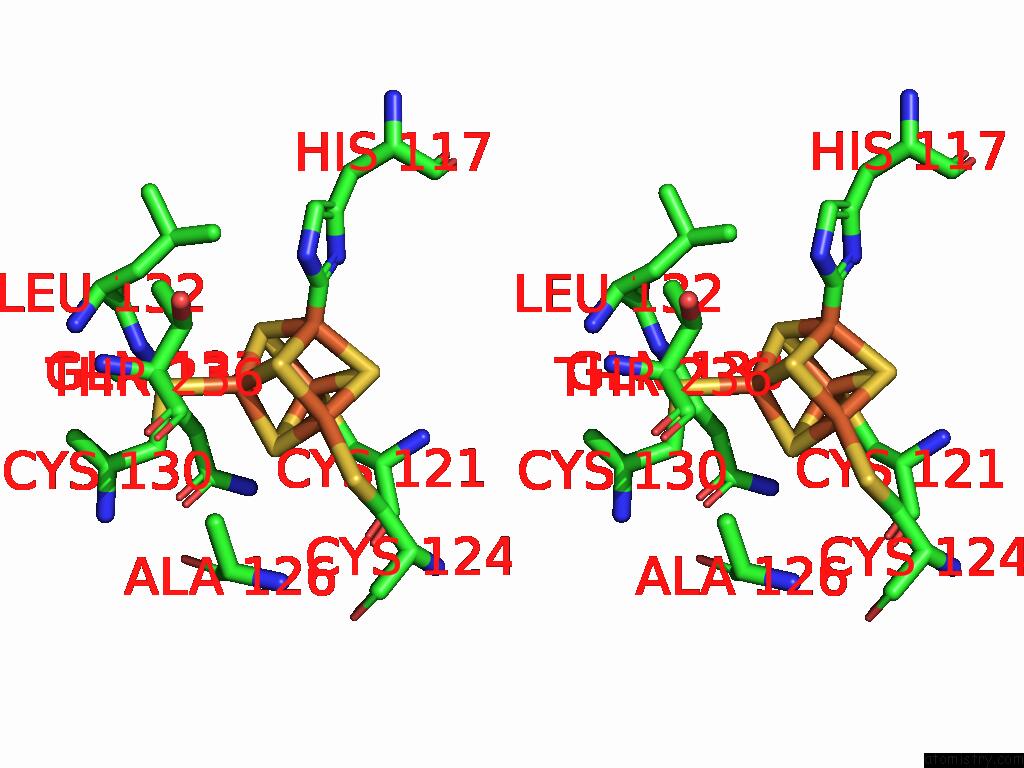
Iron binding site 3 out of 48 in 9ktl
Go back to
Iron binding site 3 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
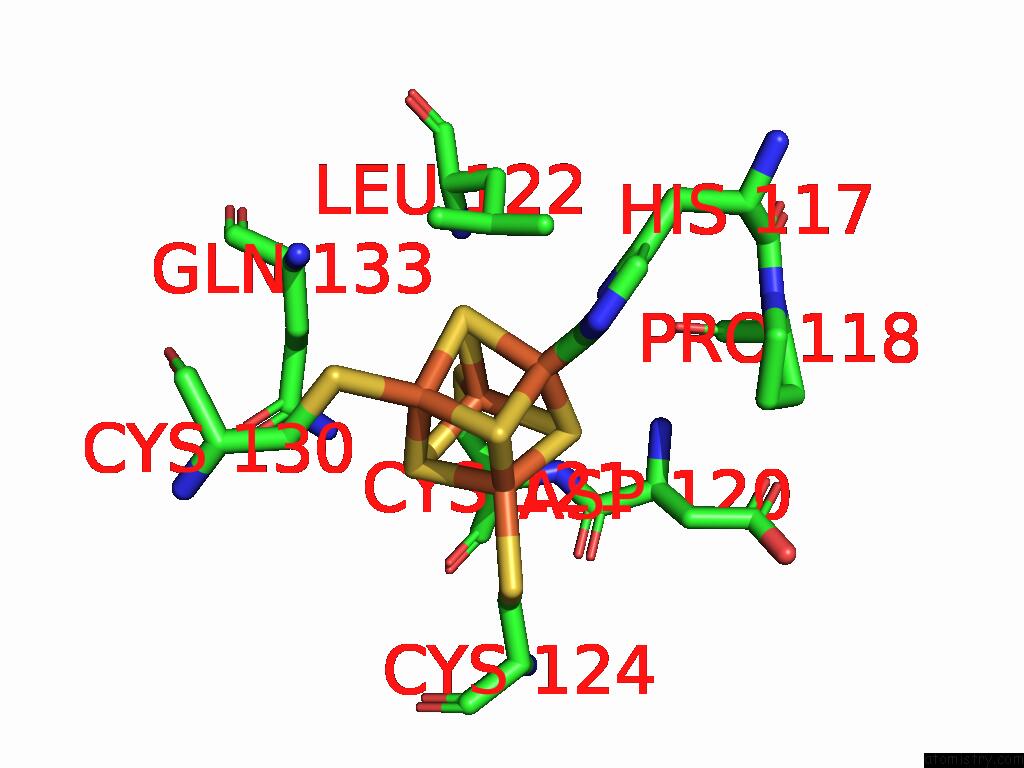
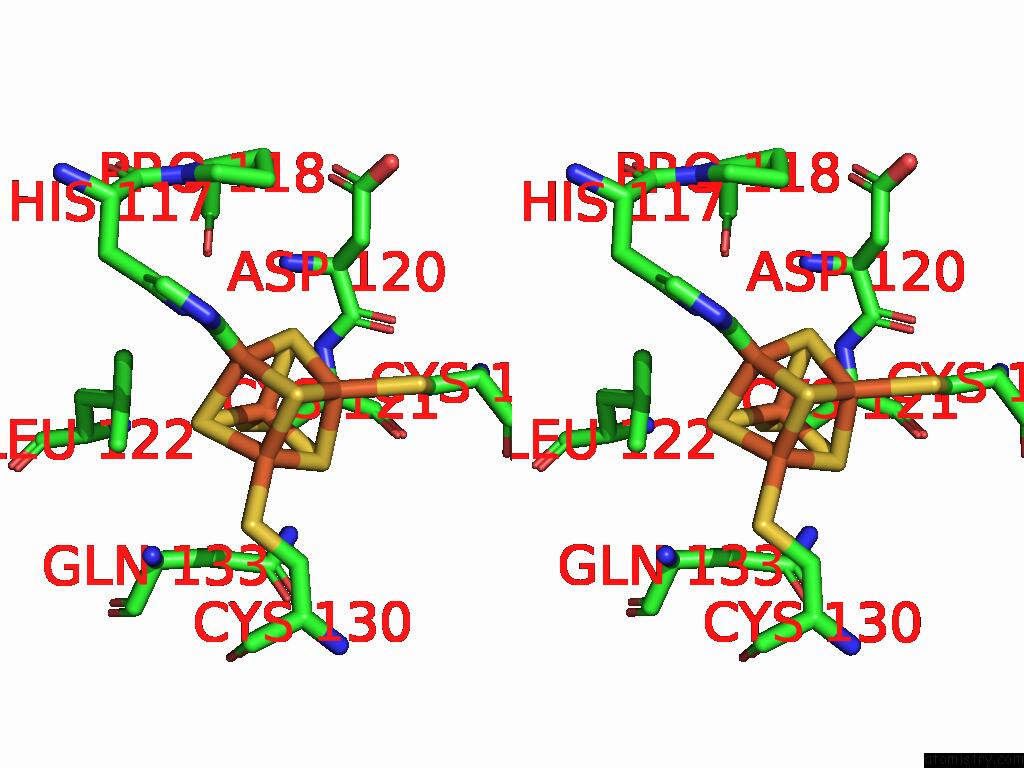
Iron binding site 4 out of 48 in 9ktl
Go back to
Iron binding site 4 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
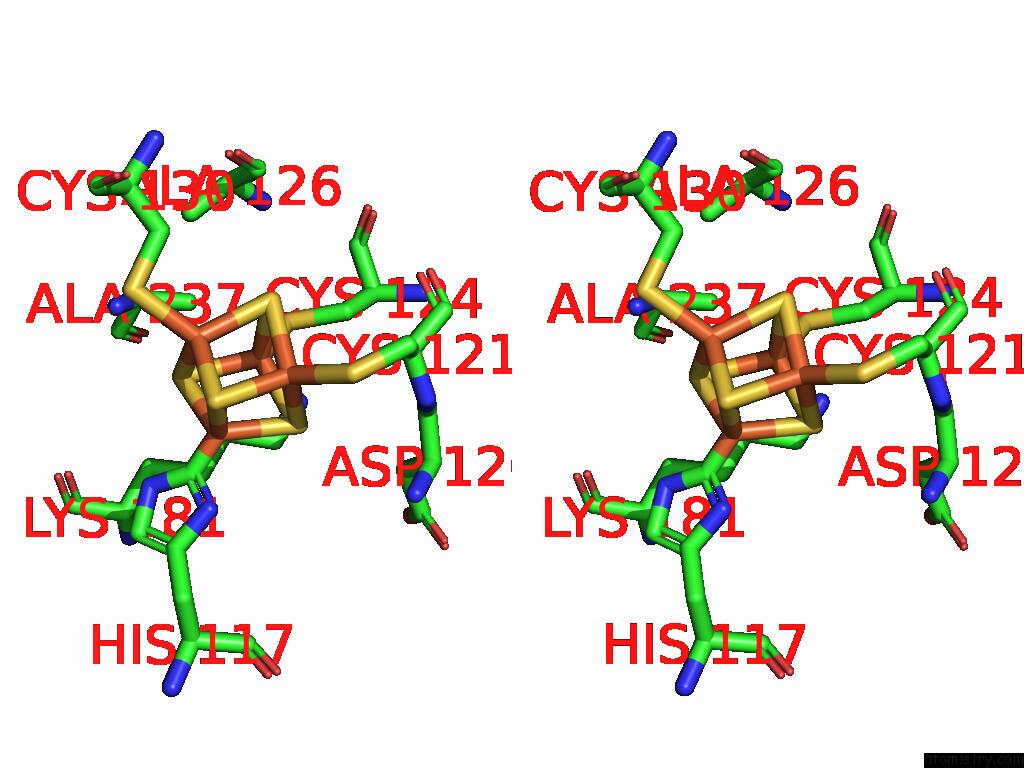
Iron binding site 5 out of 48 in 9ktl
Go back to
Iron binding site 5 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
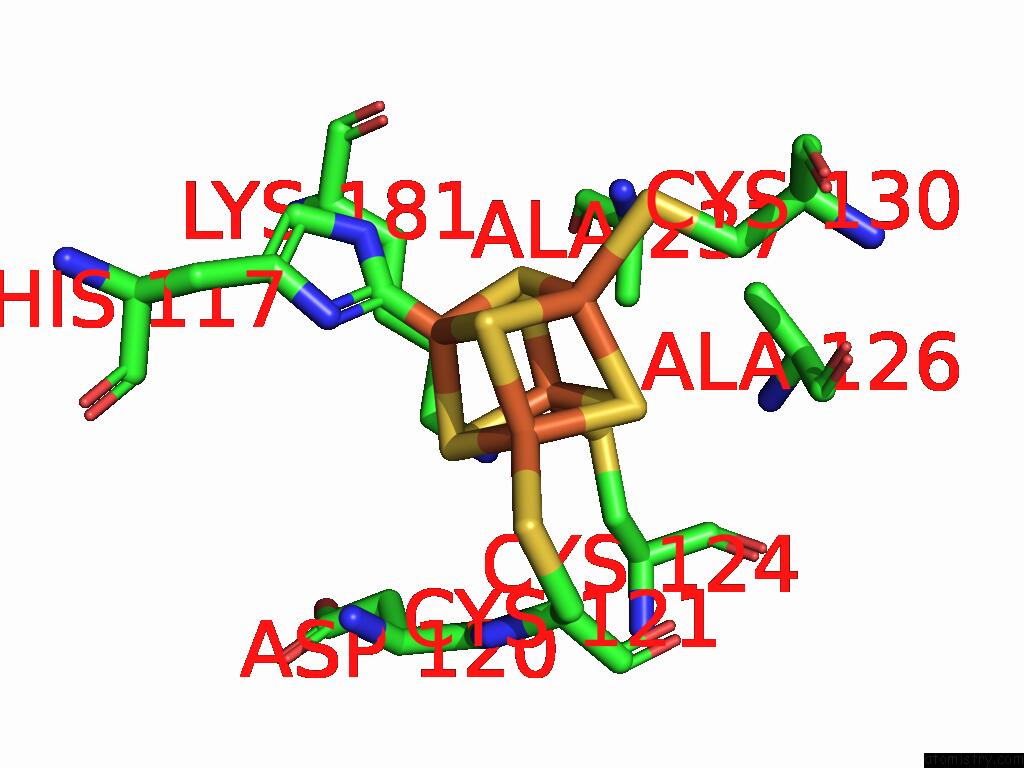
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
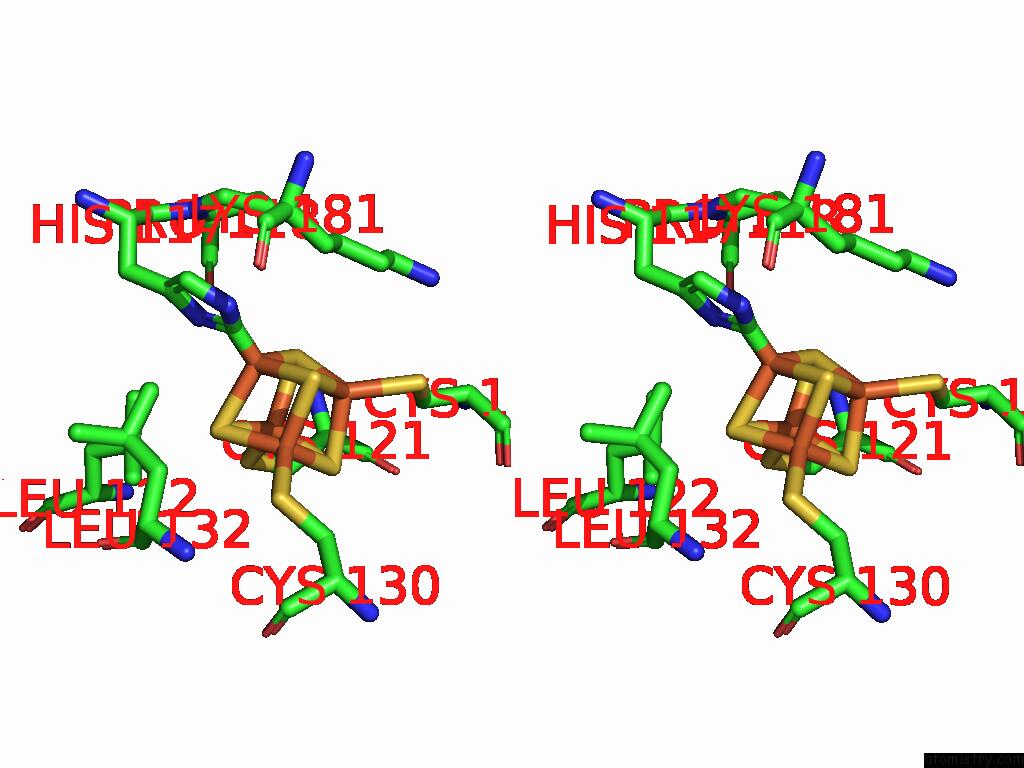
Iron binding site 6 out of 48 in 9ktl
Go back to
Iron binding site 6 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
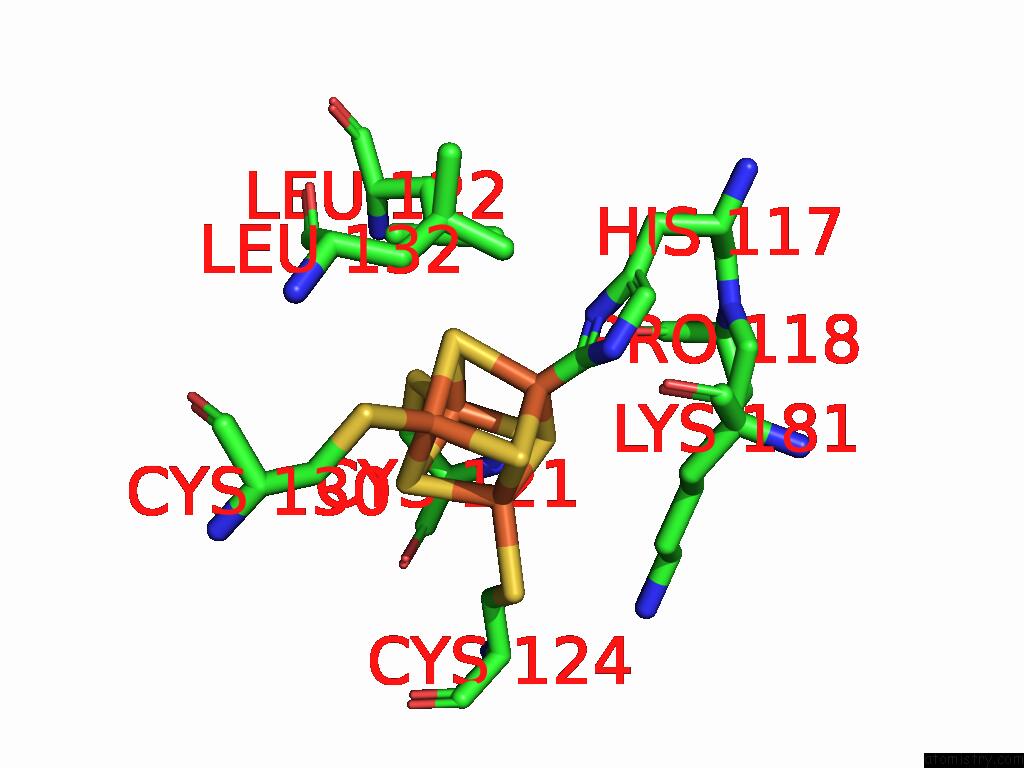
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
Iron binding site 7 out of 48 in 9ktl
Go back to
Iron binding site 7 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
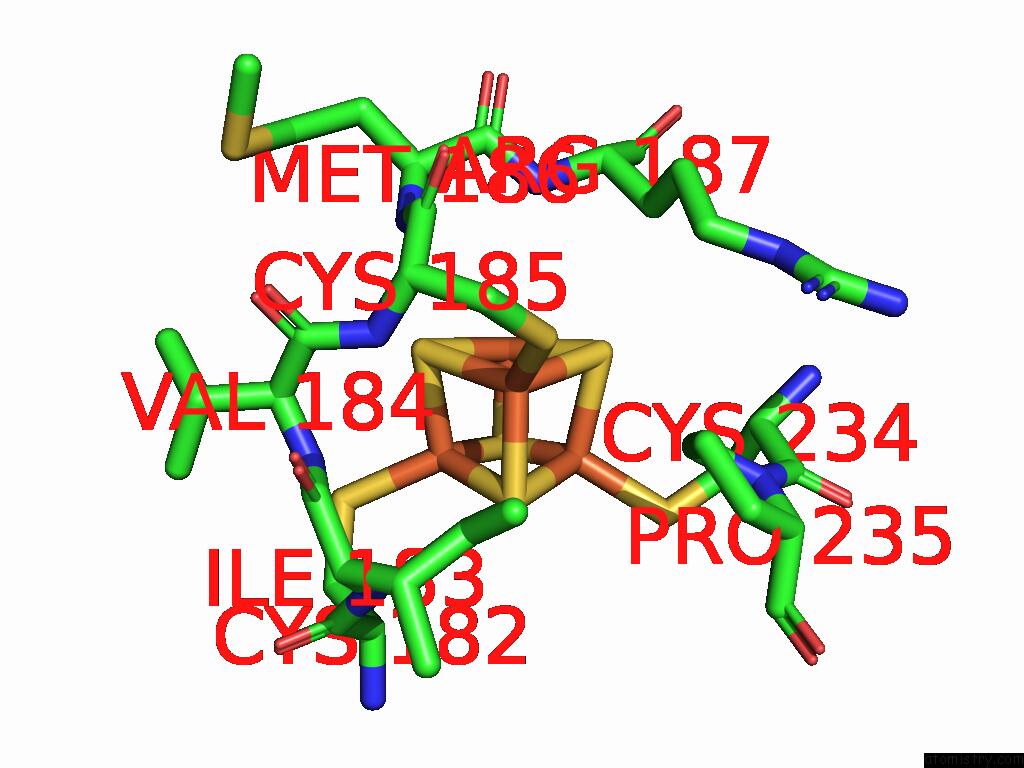
Mono view
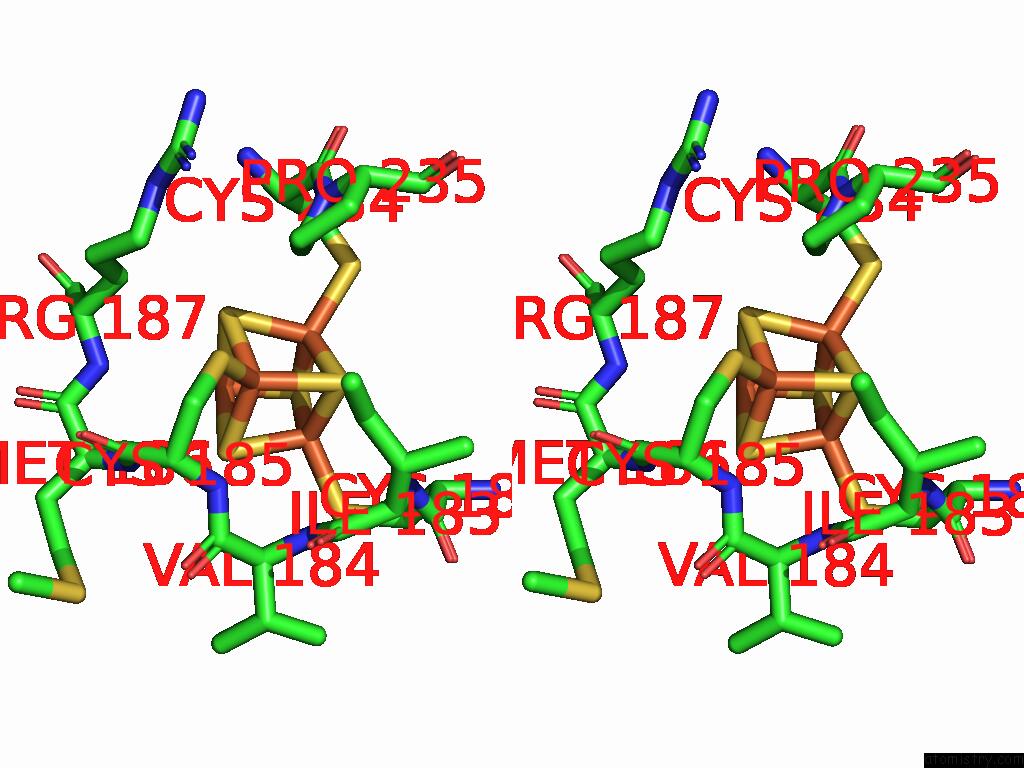
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
Iron binding site 8 out of 48 in 9ktl
Go back to
Iron binding site 8 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
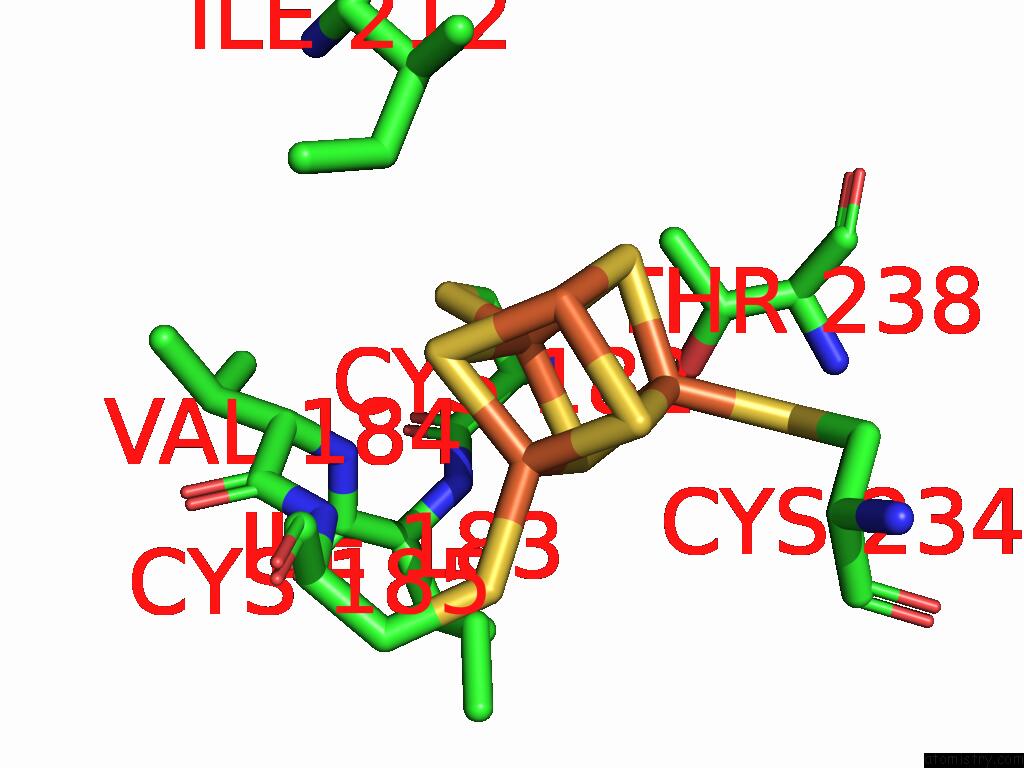
Mono view
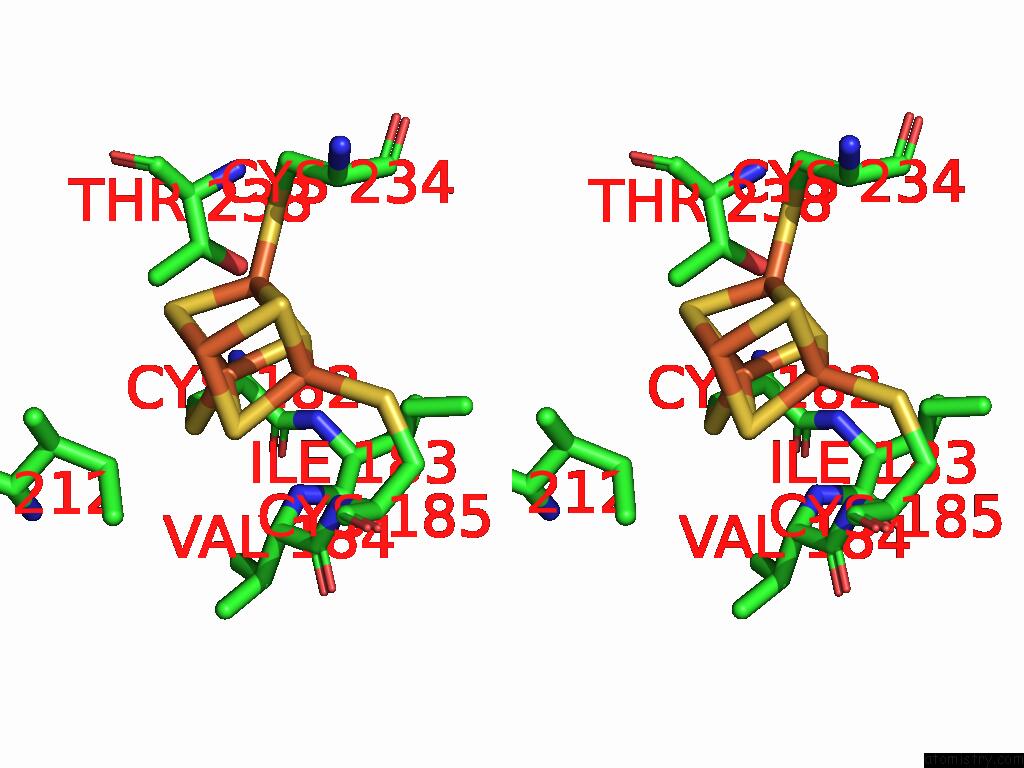
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
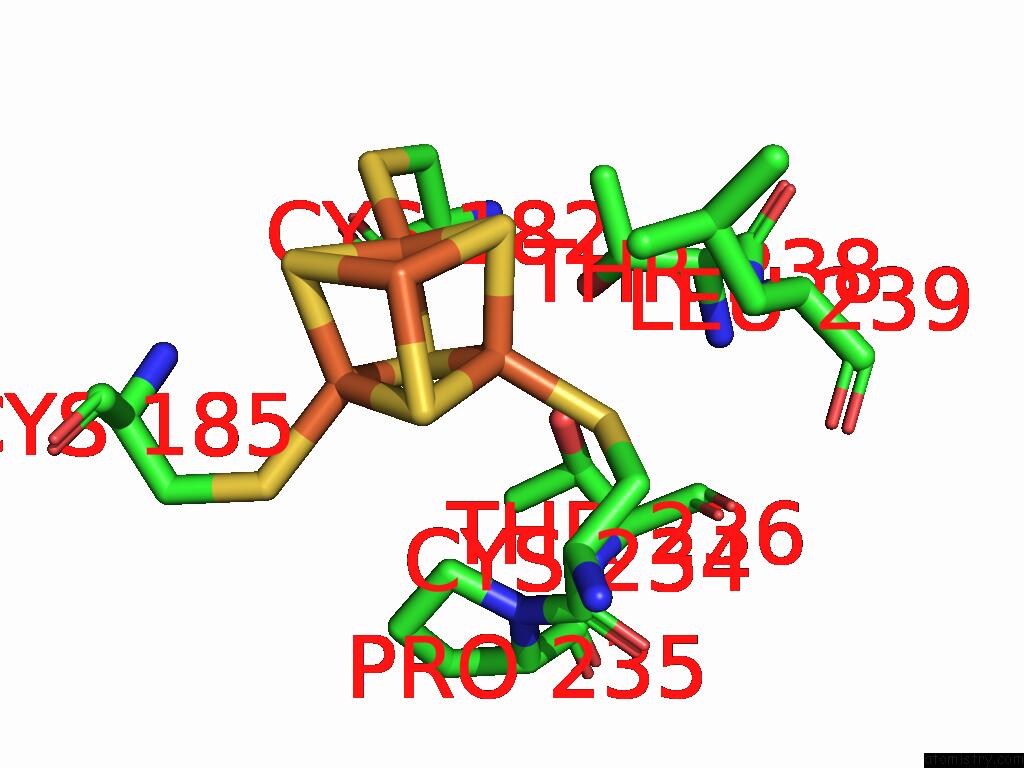
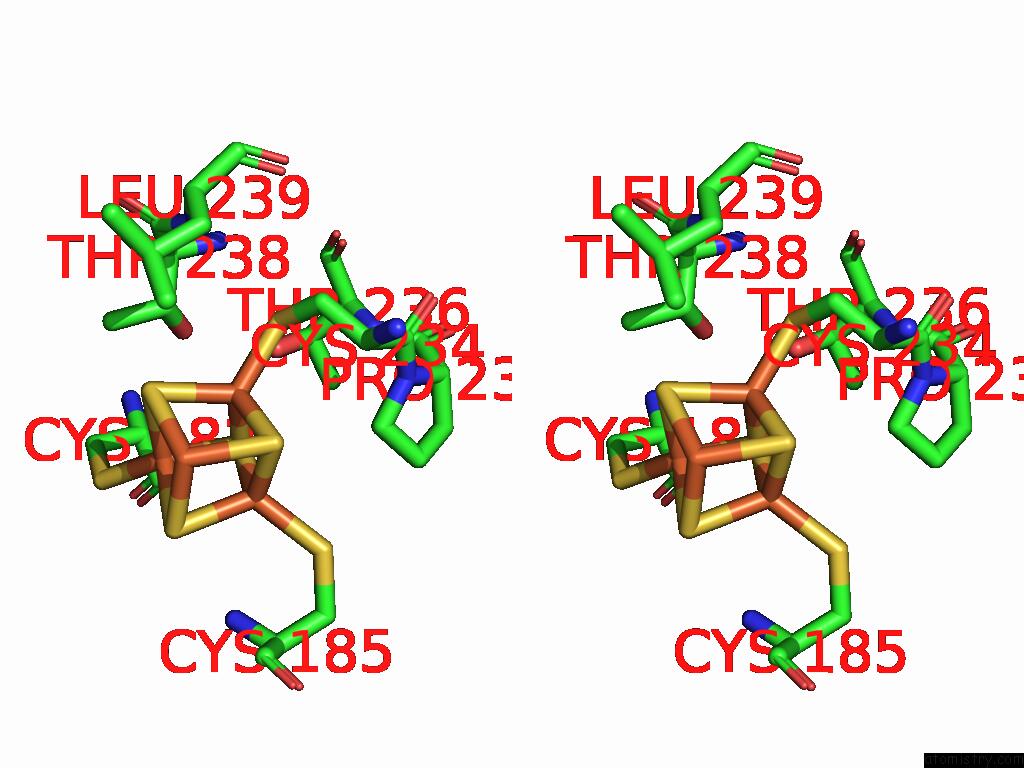
Iron binding site 9 out of 48 in 9ktl
Go back to
Iron binding site 9 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
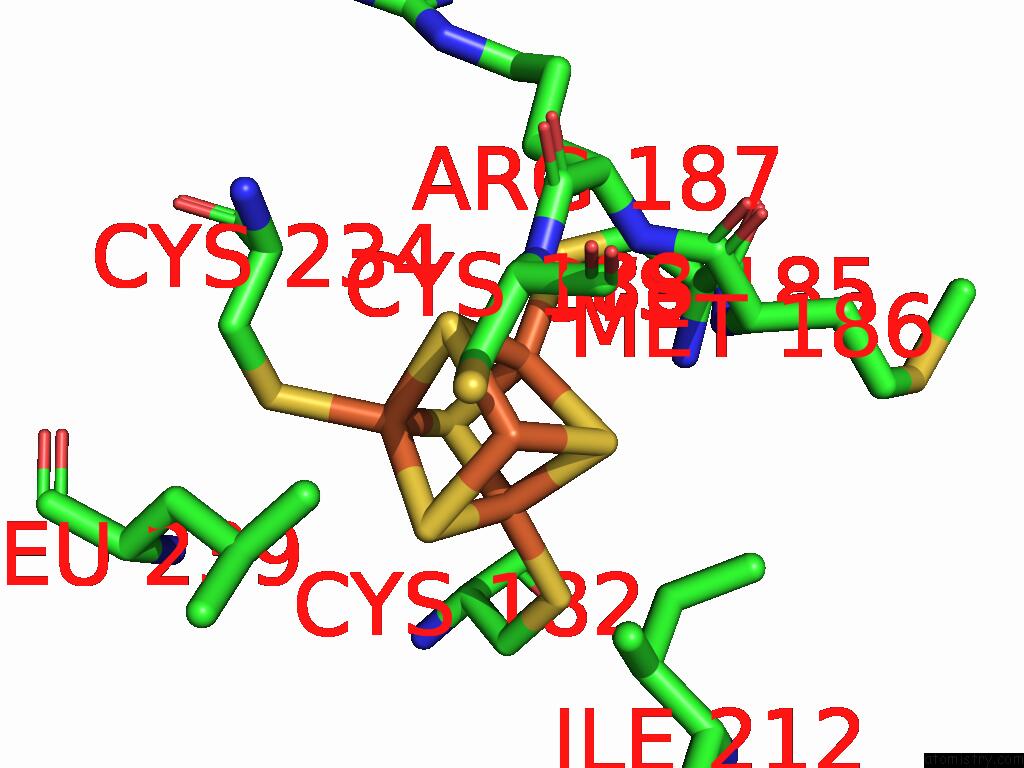
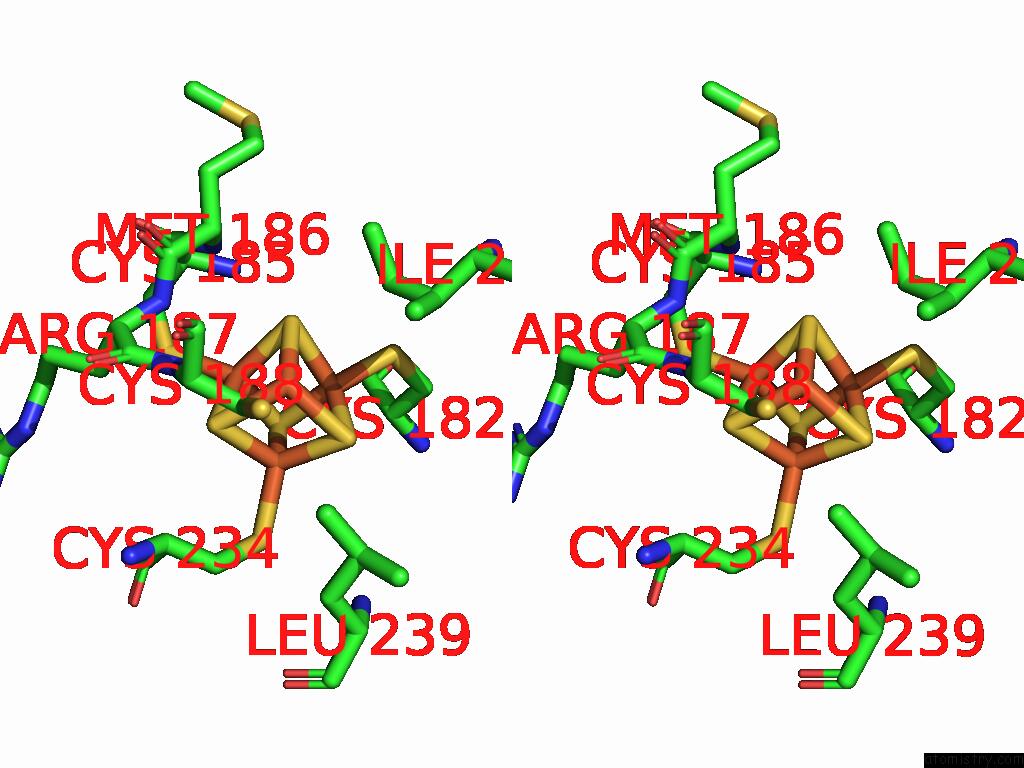
Iron binding site 10 out of 48 in 9ktl
Go back to
Iron binding site 10 out
of 48 in the Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Cryo-Em Structure of Reduced Form of Formate Dehydrogenase From Rhodobacter Aestuarii (Rafdh) with Nadh within 5.0Å range:
|
Reference:
K.Zhang,
L.Zhang.
Mechanistic Understanding on the Catalytic Preference of Formate Dehydrogenase From Rhodobacter Aestuarii Towards Carbon Dioxide Reduction To Be Published.
Page generated: Fri Aug 8 07:08:15 2025
Last articles
K in 9G4QK in 9FYE
K in 9FT7
K in 9FQ1
K in 9FM9
K in 9EX3
K in 9F90
K in 9ES6
K in 9EWD
K in 9ETN