Iron »
PDB 1dj7-1dry »
1dlm »
Iron in PDB 1dlm: Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data
Enzymatic activity of Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data
All present enzymatic activity of Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data:
1.13.11.1;
1.13.11.1;
Protein crystallography data
The structure of Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data, PDB code: 1dlm
was solved by
M.W.Vetting,
D.H.Ohlendorf,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 52.600, 87.600, 84.200, 90.00, 96.30, 90.00 |
| R / Rfree (%) | 19.6 / 24.3 |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data
(pdb code 1dlm). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data, PDB code: 1dlm:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data, PDB code: 1dlm:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 1dlm
Go back to
Iron binding site 1 out
of 2 in the Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data
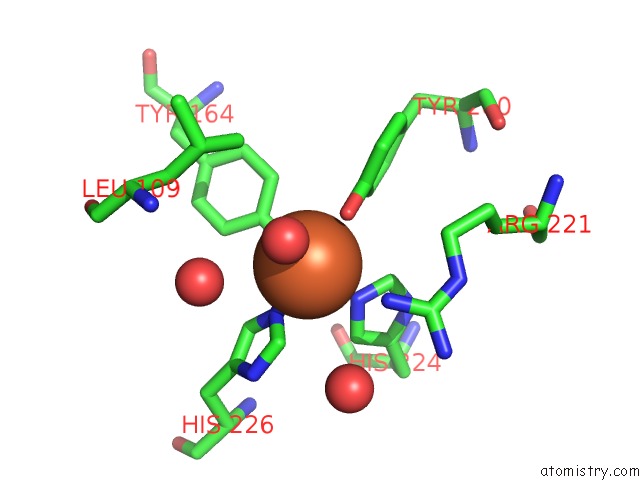
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data within 5.0Å range:
|
Iron binding site 2 out of 2 in 1dlm
Go back to
Iron binding site 2 out
of 2 in the Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data

Mono view
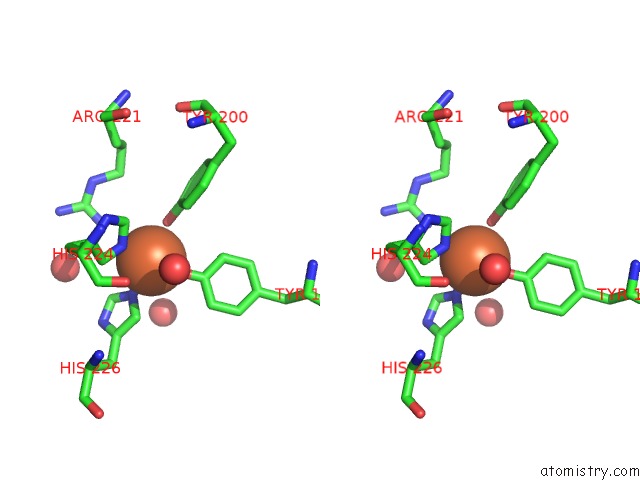
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Catechol 1,2-Dioxygenase From Acinetobacter Calcoaceticus Native Data within 5.0Å range:
|
Reference:
M.W.Vetting,
D.H.Ohlendorf.
The 1.8 A Crystal Structure of Catechol 1,2-Dioxygenase Reveals A Novel Hydrophobic Helical Zipper As A Subunit Linker. Structure Fold.Des. V. 8 429 2000.
ISSN: ISSN 0969-2126
PubMed: 10801478
DOI: 10.1016/S0969-2126(00)00122-2
Page generated: Sat Aug 3 03:48:37 2024
ISSN: ISSN 0969-2126
PubMed: 10801478
DOI: 10.1016/S0969-2126(00)00122-2
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW