Iron »
PDB 1jl6-1k2o »
1jwn »
Iron in PDB 1jwn: Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
Protein crystallography data
The structure of Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide., PDB code: 1jwn
was solved by
J.E.Knapp,
Q.H.Gibson,
L.Cushing,
W.E.Royer Jr.,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.50 / 2.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 83.680, 44.140, 87.280, 90.00, 115.42, 90.00 |
| R / Rfree (%) | 21.1 / 24.7 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
(pdb code 1jwn). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide., PDB code: 1jwn:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide., PDB code: 1jwn:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 1jwn
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
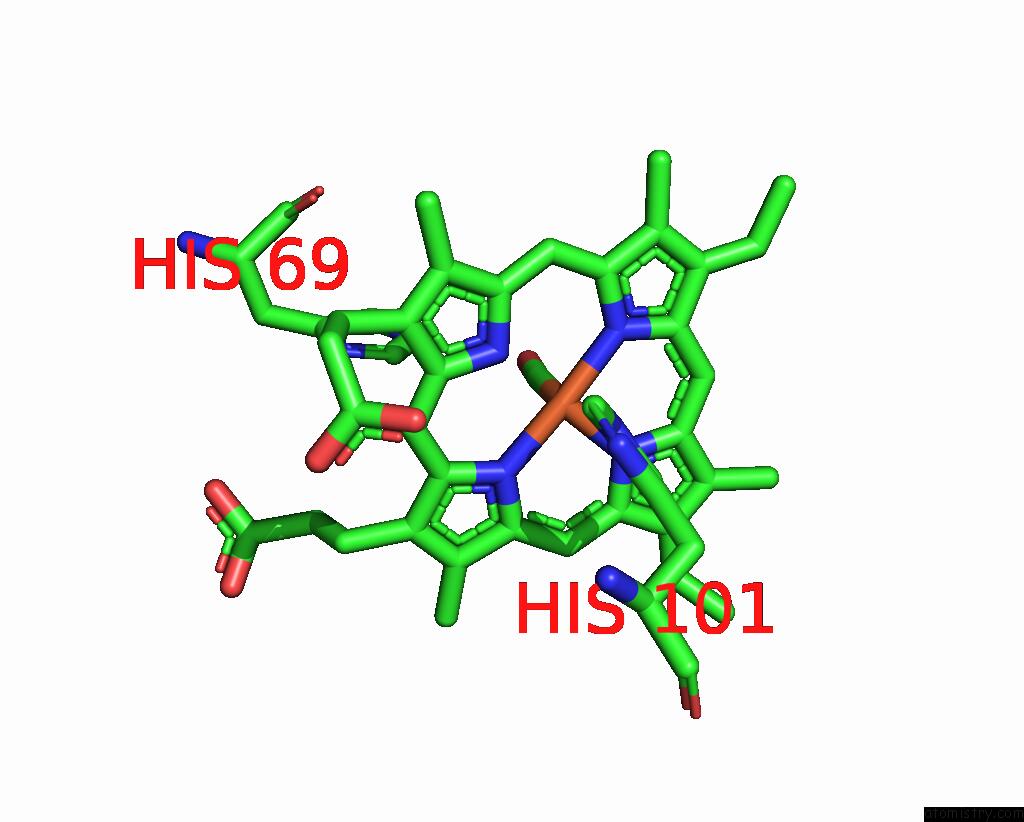
Mono view
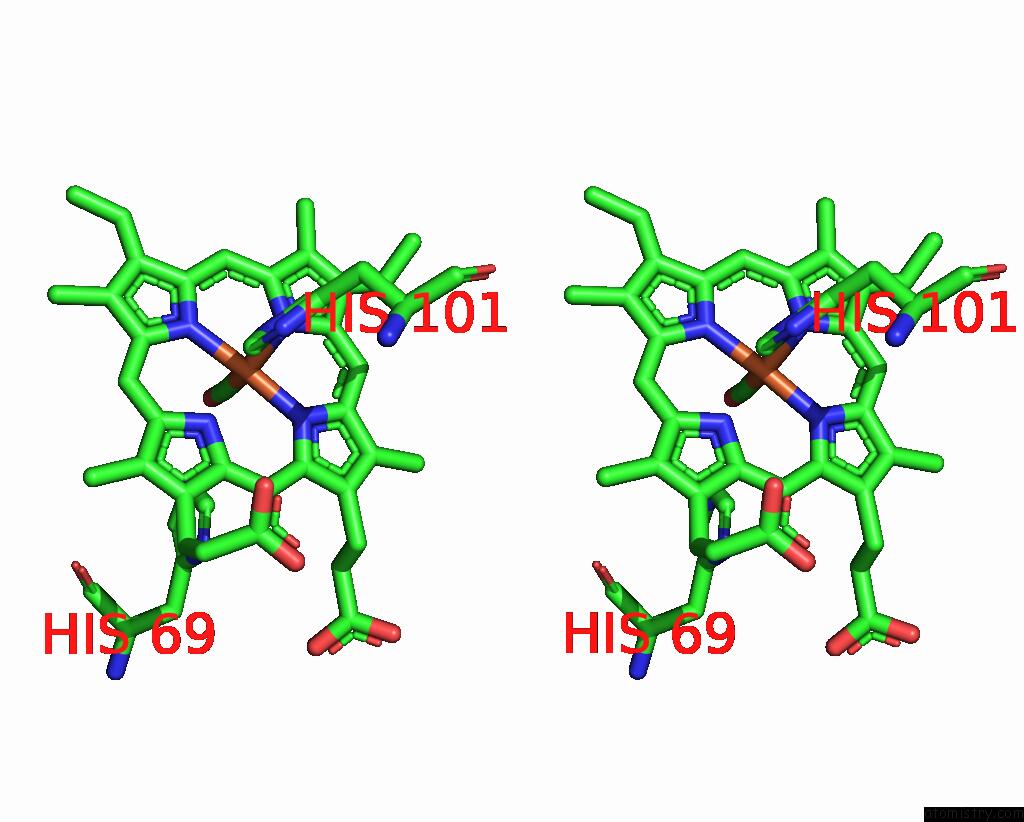
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide. within 5.0Å range:
|
Iron binding site 2 out of 4 in 1jwn
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
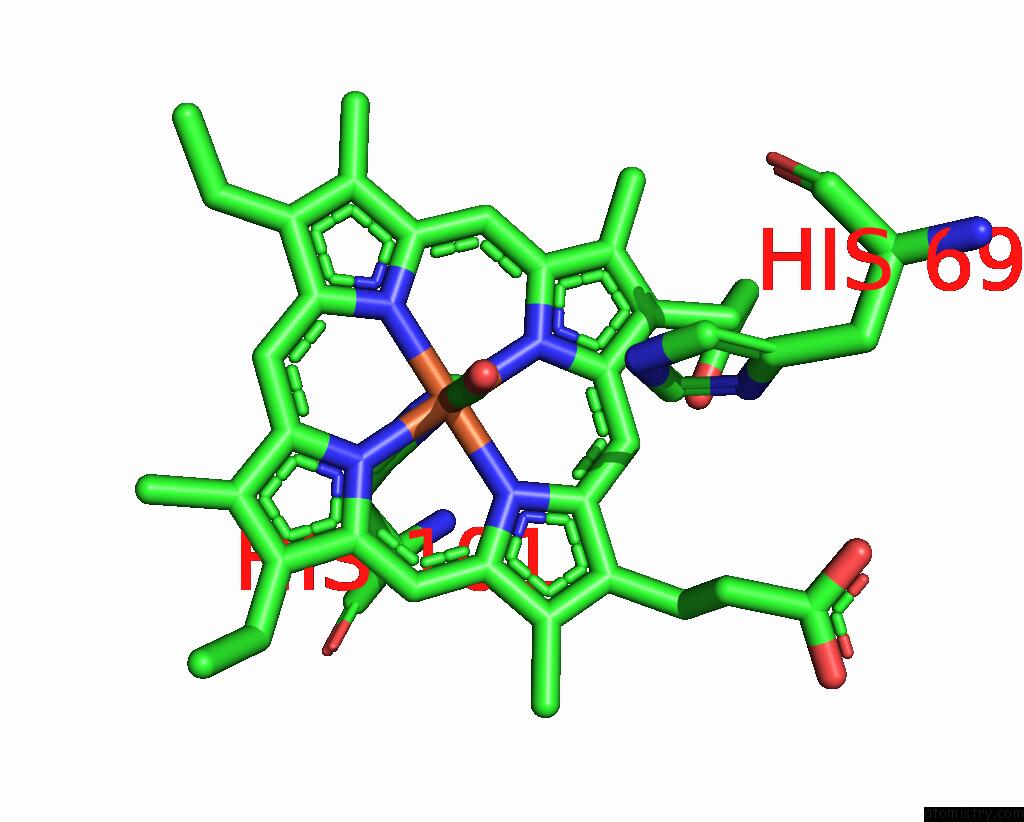
Mono view
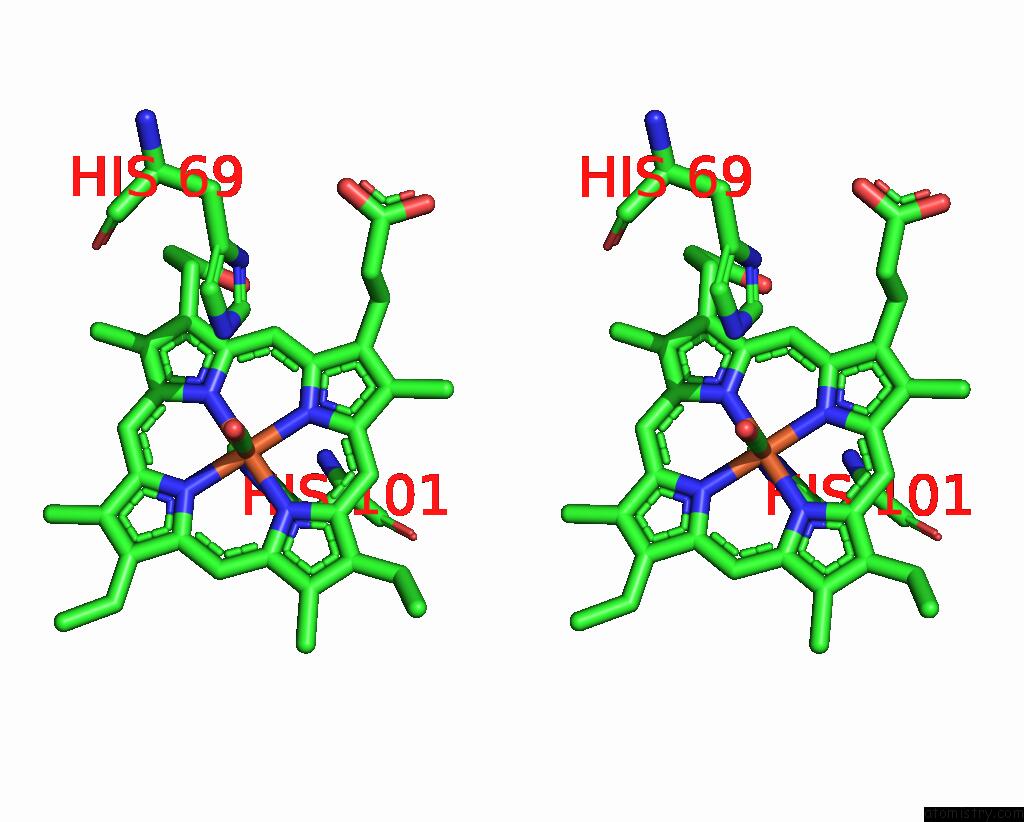
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide. within 5.0Å range:
|
Iron binding site 3 out of 4 in 1jwn
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
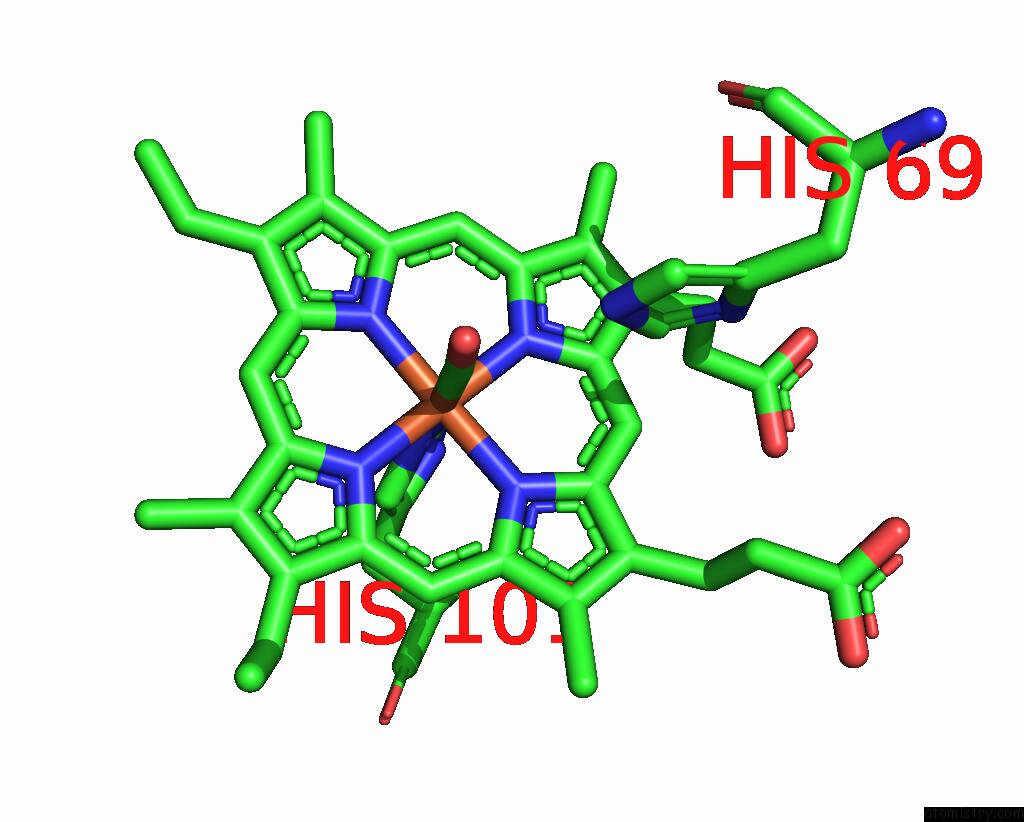
Mono view
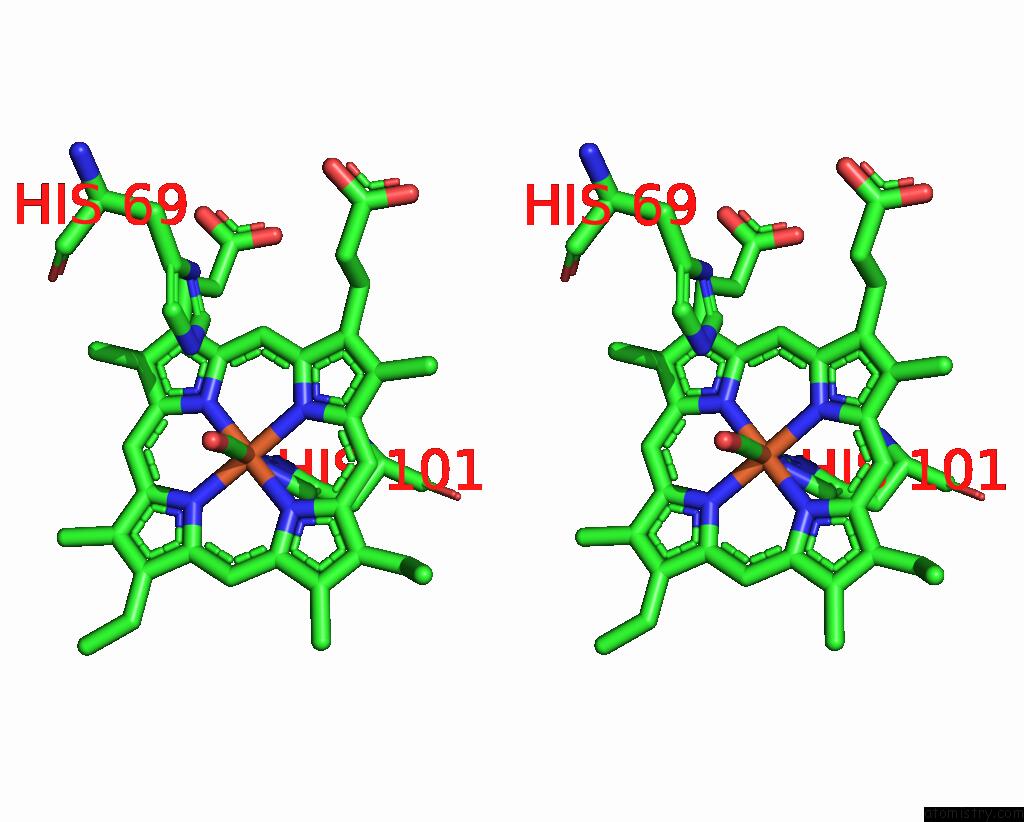
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide. within 5.0Å range:
|
Iron binding site 4 out of 4 in 1jwn
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide.
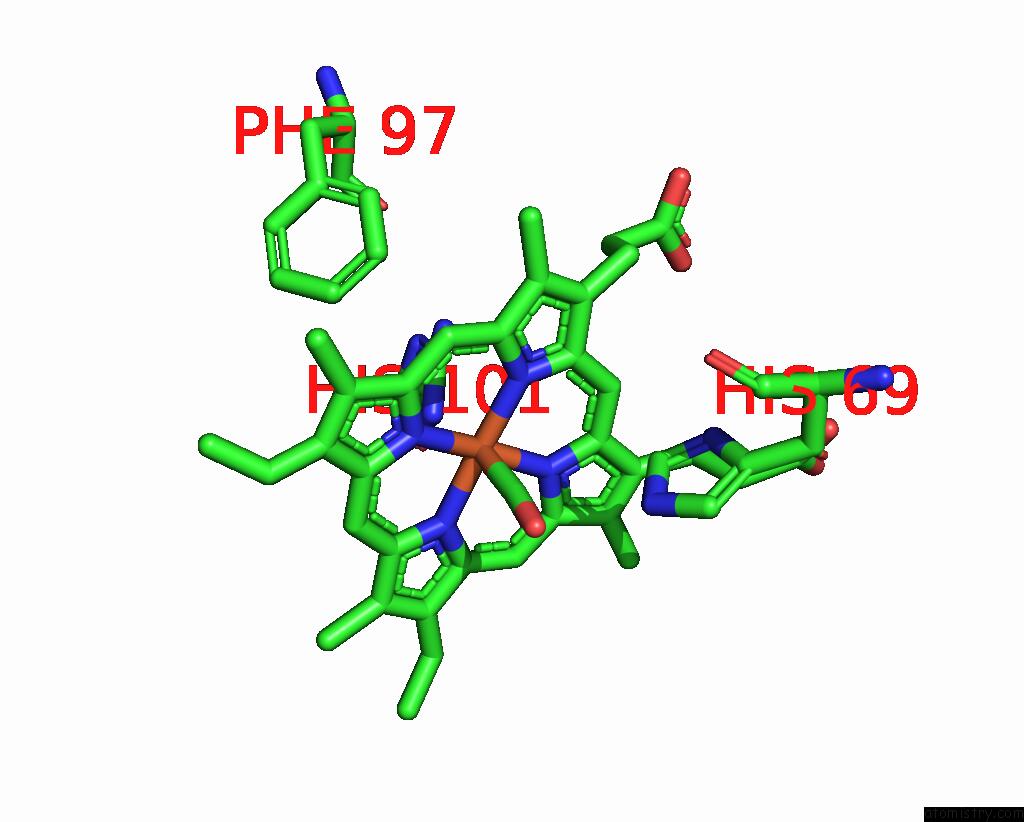
Mono view
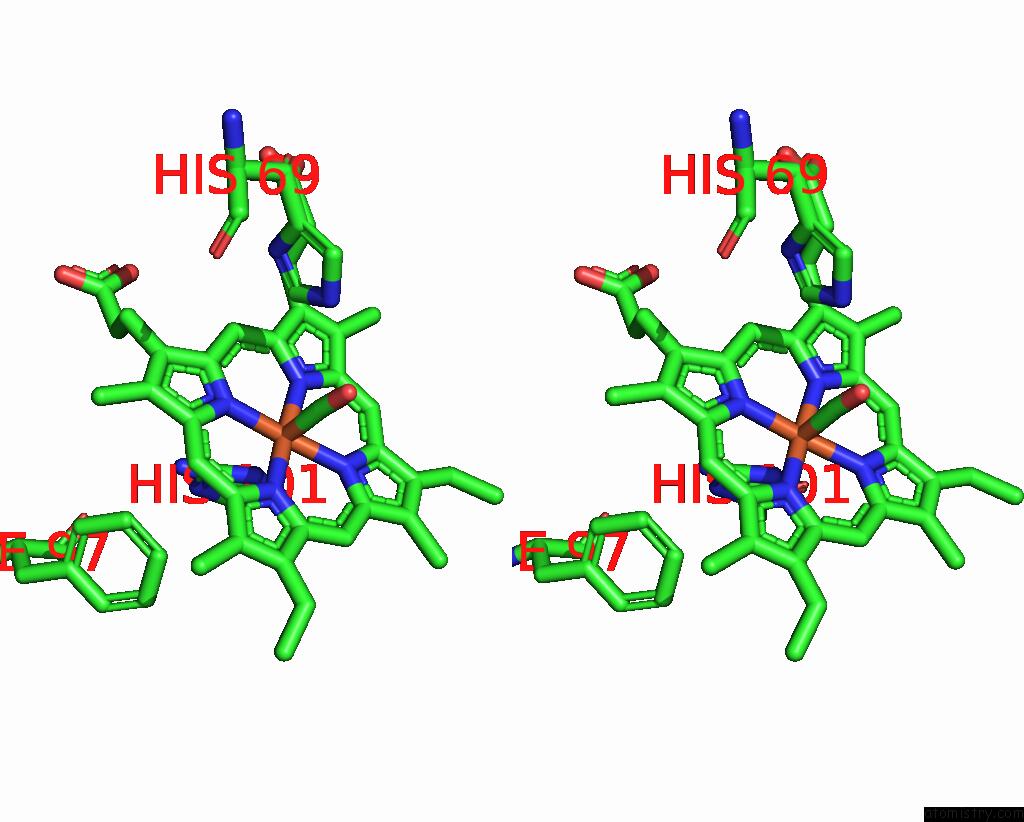
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of Scapharca Inaequivalvis Hbi, I114F Mutant Ligated to Carbon Monoxide. within 5.0Å range:
|
Reference:
J.E.Knapp,
Q.H.Gibson,
L.Cushing,
W.E.Royer Jr..
Restricting the Ligand-Linked Heme Movement in Scapharca Dimeric Hemoglobin Reveals Tight Coupling Between Distal and Proximal Contributions to Cooperativity. Biochemistry V. 40 14795 2001.
ISSN: ISSN 0006-2960
PubMed: 11732898
DOI: 10.1021/BI011071T
Page generated: Sat Aug 3 08:47:38 2024
ISSN: ISSN 0006-2960
PubMed: 11732898
DOI: 10.1021/BI011071T
Last articles
F in 7OHNF in 7OIH
F in 7OHL
F in 7OH6
F in 7OHK
F in 7OHH
F in 7OH5
F in 7OFK
F in 7OFI
F in 7OAR