Iron »
PDB 1lrm-1m6m »
1ltw »
Iron in PDB 1ltw: Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy)
Protein crystallography data
The structure of Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy), PDB code: 1ltw
was solved by
T.Li,
J.S.Olson,
G.N.Phillips Jr.,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 5.00 / 1.70 |
| Space group | P 6 |
| Cell size a, b, c (Å), α, β, γ (°) | 91.461, 91.461, 45.841, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 15.8 / n/a |
Iron Binding Sites:
The binding sites of Iron atom in the Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy)
(pdb code 1ltw). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy), PDB code: 1ltw:
In total only one binding site of Iron was determined in the Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy), PDB code: 1ltw:
Iron binding site 1 out of 1 in 1ltw
Go back to
Iron binding site 1 out
of 1 in the Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy)
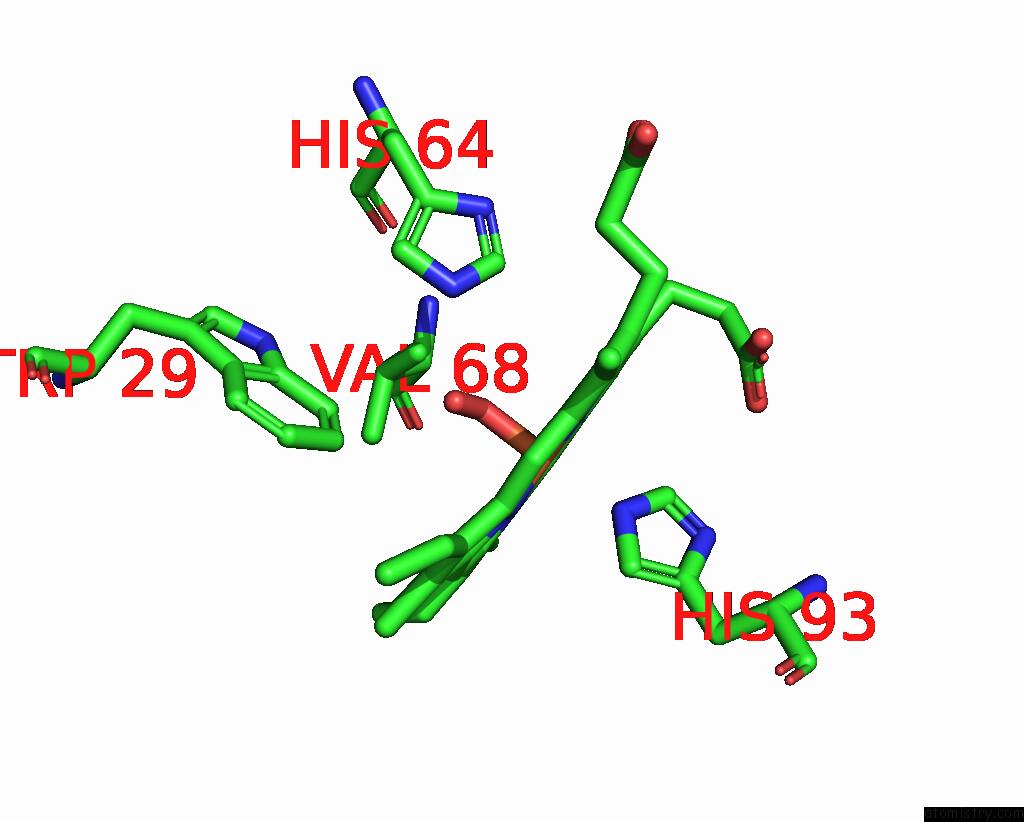
Mono view
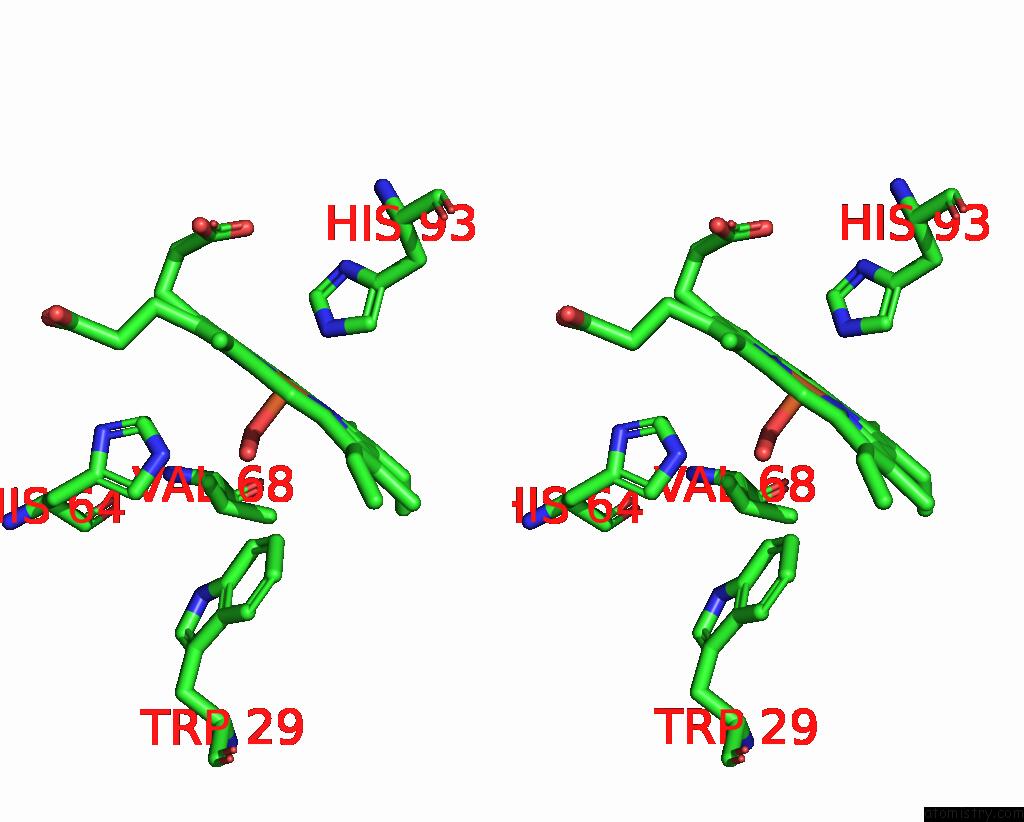
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Recombinant Sperm Whale Myoglobin 29W Mutant (Oxy) within 5.0Å range:
|
Reference:
S.Hirota,
T.Li,
G.N.Phillips Jr.,
J.S.Olson,
M.Mukai,
T.Kitagawa.
Perturbation of the Fe-O2 Bond By Nearby Residues in Heme Pocket: Observation of Vfe-O2 Raman Bands For Oxymyoglobin Mutants J.Am.Chem.Soc. V. 118 7845 1996.
ISSN: ISSN 0002-7863
Page generated: Sat Aug 3 09:55:17 2024
ISSN: ISSN 0002-7863
Last articles
F in 7QMOF in 7QMN
F in 7QMM
F in 7QML
F in 7QMK
F in 7QMJ
F in 7QMI
F in 7QMH
F in 7QMG
F in 7QMF