Iron »
PDB 1ofj-1ozl »
1oqu »
Iron in PDB 1oqu: A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
Protein crystallography data
The structure of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes, PDB code: 1oqu
was solved by
M.Hogbom,
P.Nordlund,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 49.319, 91.237, 136.956, 90.00, 91.46, 90.00 |
| R / Rfree (%) | 18.1 / 23.9 |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20;Binding sites:
The binding sites of Iron atom in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes (pdb code 1oqu). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 20 binding sites of Iron where determined in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes, PDB code: 1oqu:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 20 in 1oqu
Go back to
Iron binding site 1 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
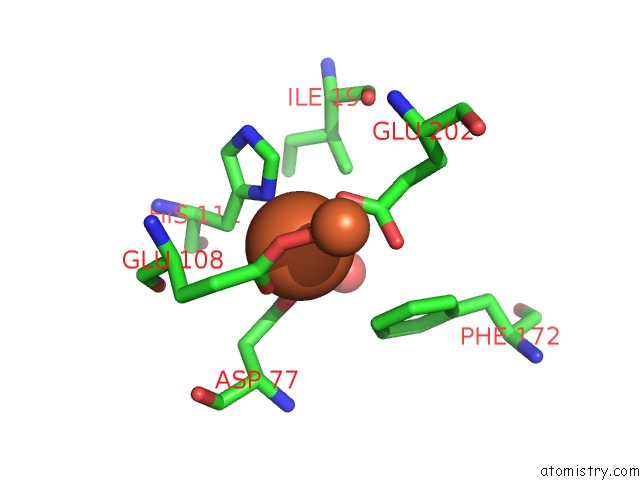
Mono view
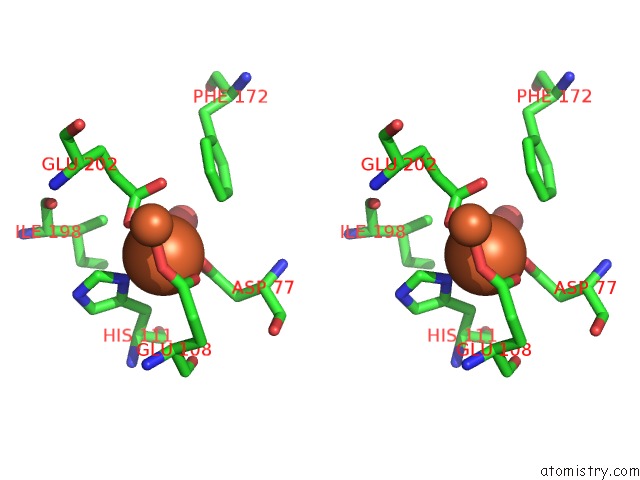
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 2 out of 20 in 1oqu
Go back to
Iron binding site 2 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
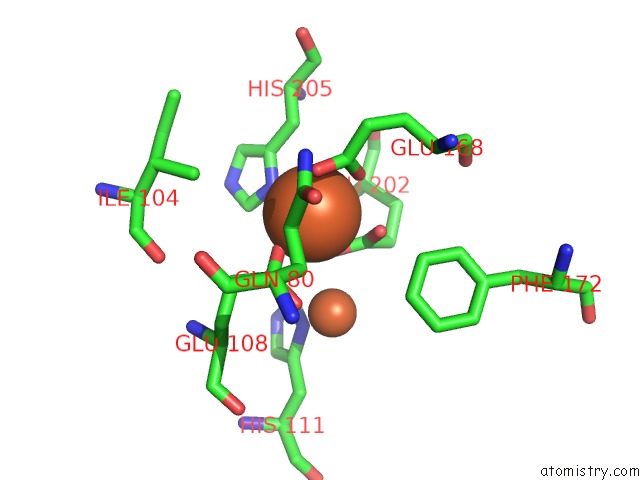
Mono view
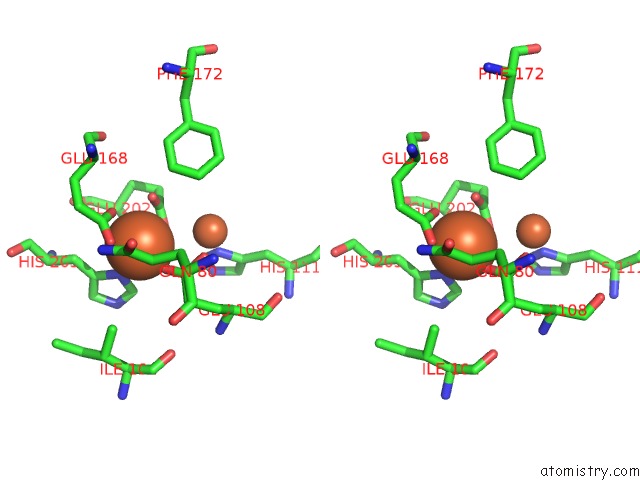
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 3 out of 20 in 1oqu
Go back to
Iron binding site 3 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
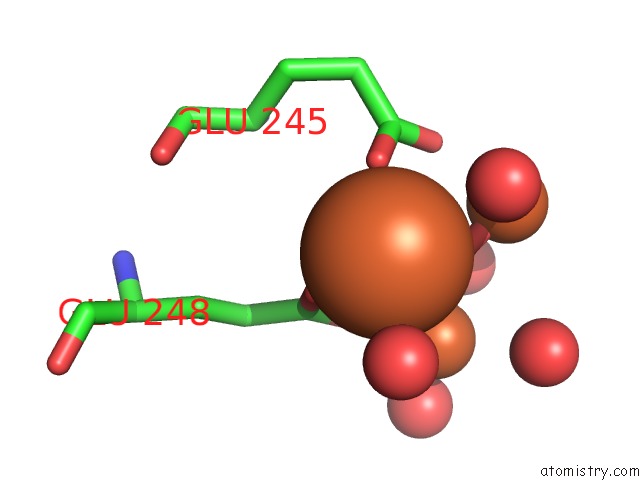
Mono view
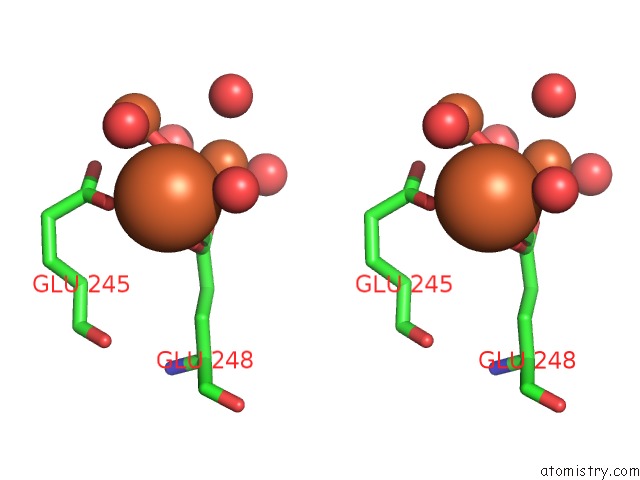
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 4 out of 20 in 1oqu
Go back to
Iron binding site 4 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
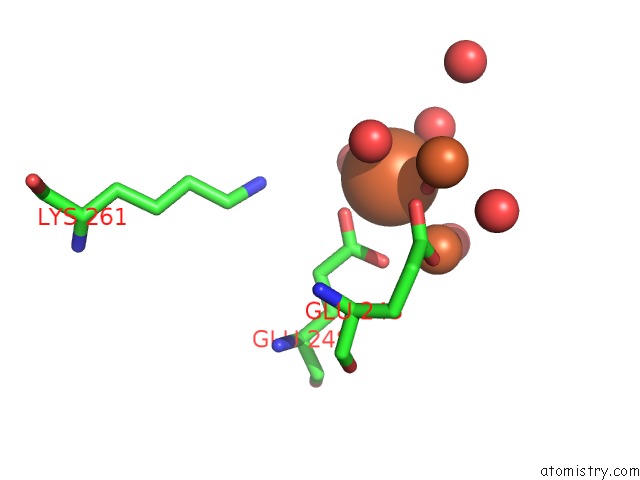
Mono view
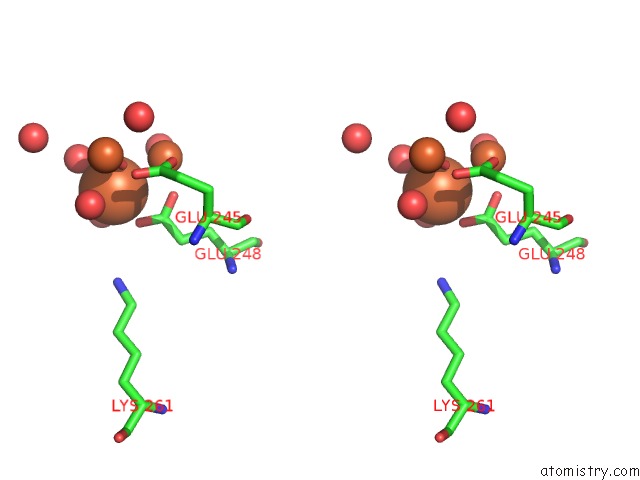
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 5 out of 20 in 1oqu
Go back to
Iron binding site 5 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
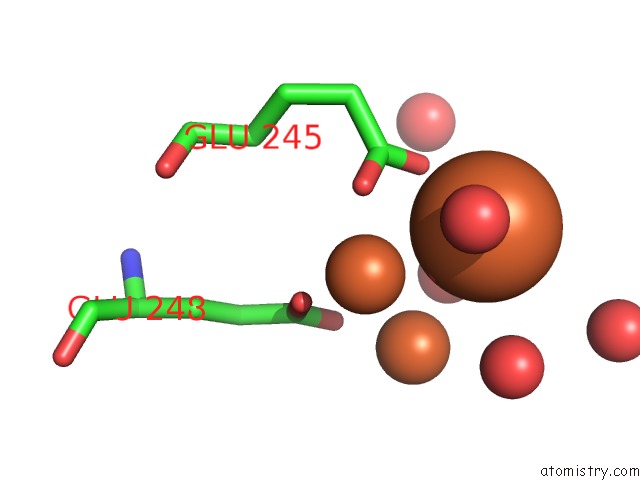
Mono view
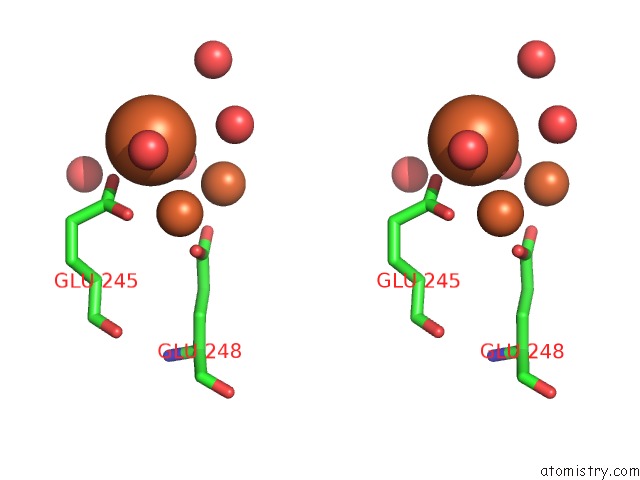
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 6 out of 20 in 1oqu
Go back to
Iron binding site 6 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
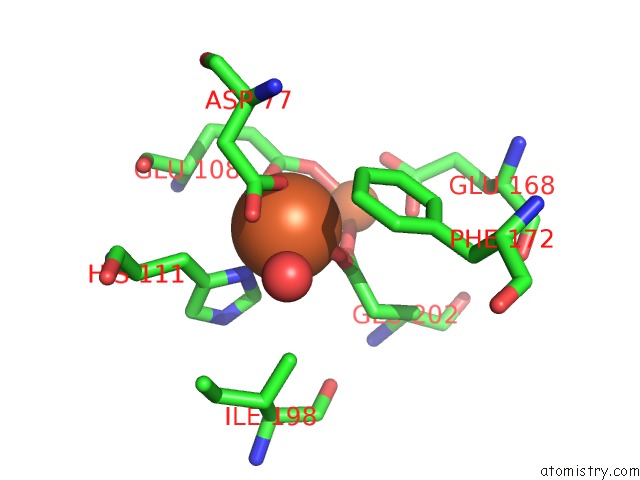
Mono view
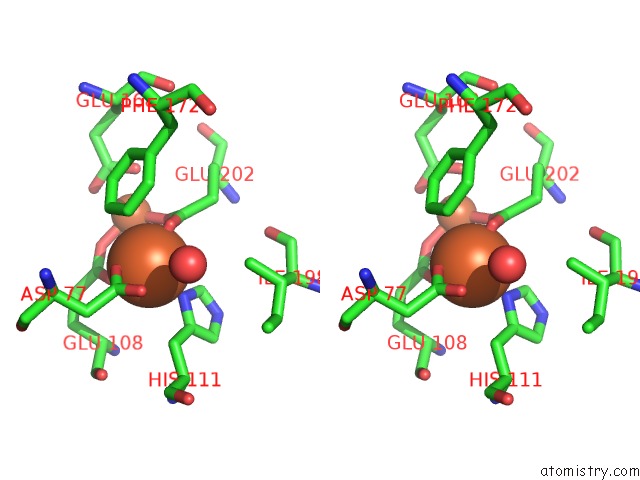
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 7 out of 20 in 1oqu
Go back to
Iron binding site 7 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
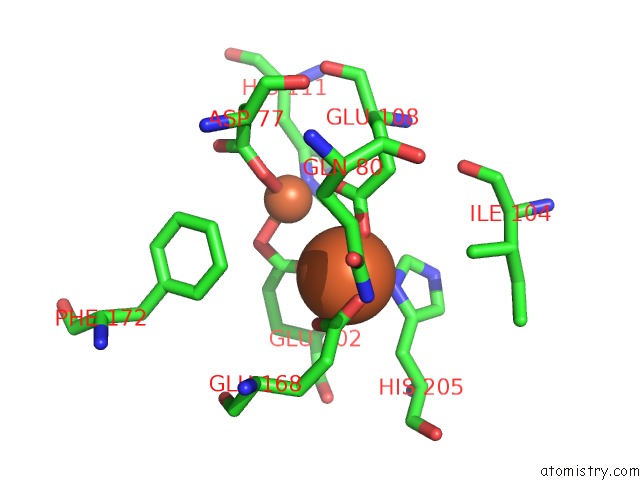
Mono view
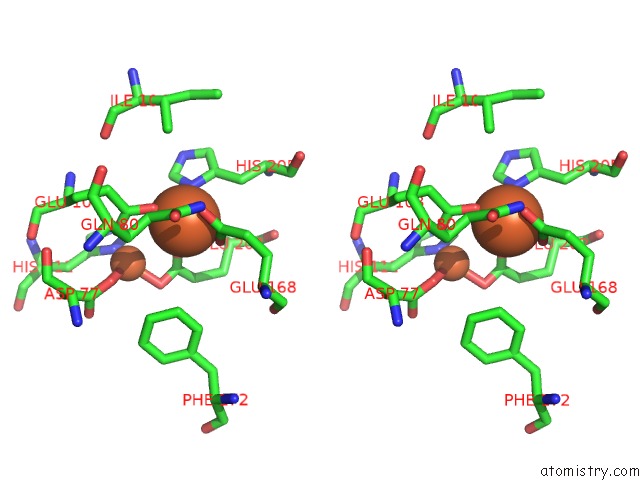
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 8 out of 20 in 1oqu
Go back to
Iron binding site 8 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
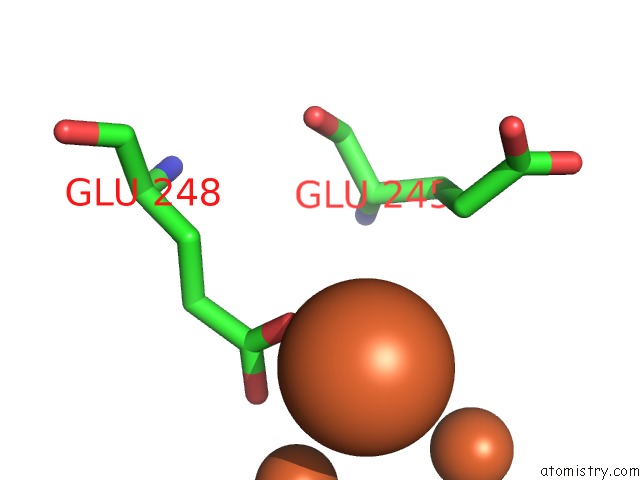
Mono view
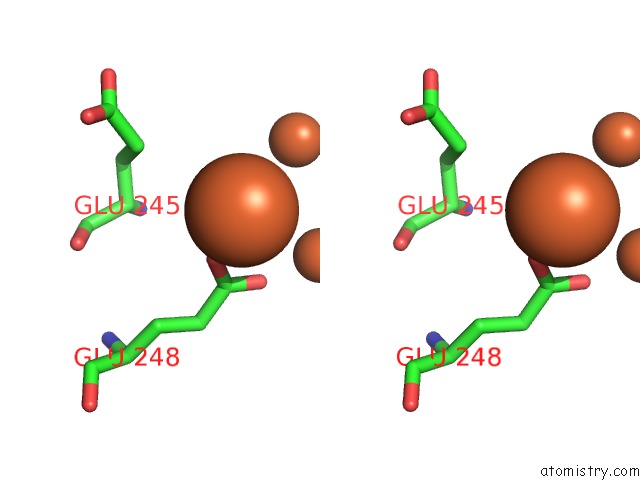
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 9 out of 20 in 1oqu
Go back to
Iron binding site 9 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
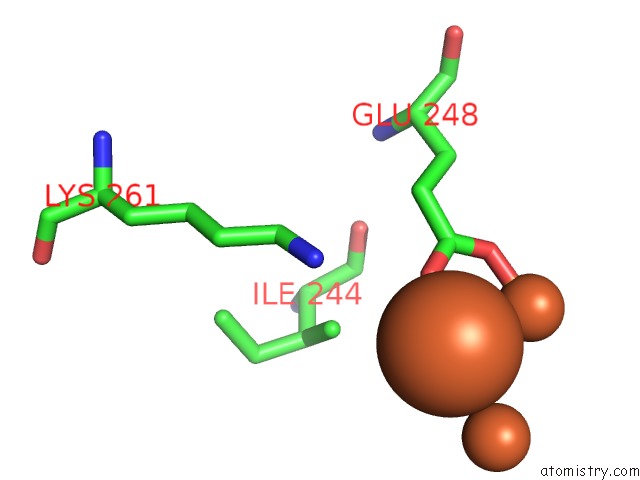
Mono view
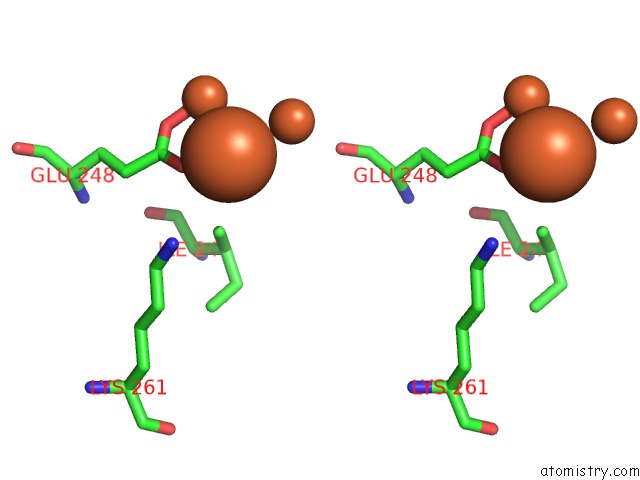
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Iron binding site 10 out of 20 in 1oqu
Go back to
Iron binding site 10 out
of 20 in the A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes
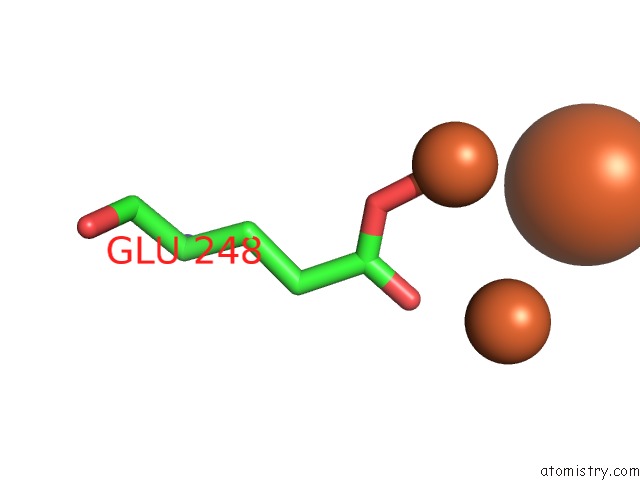
Mono view
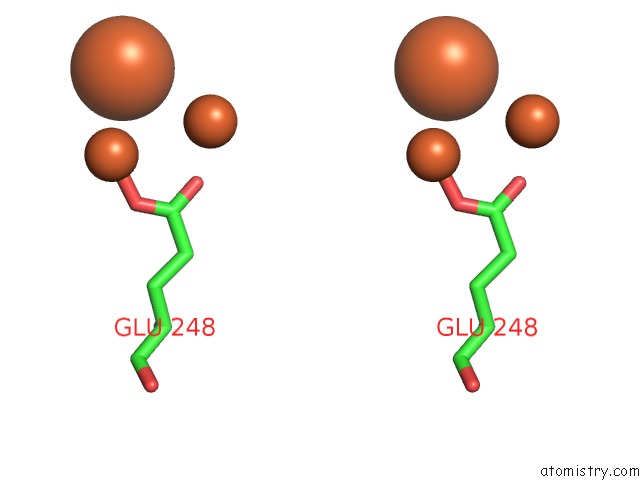
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of A Protein Coordinated Tri-Nuclear Fe Complex Formed During Soaking of Crystals of the Ribonucleotide Reductase R2F Protein From Corynebacterium Ammoniagenes within 5.0Å range:
|
Reference:
M.Hogbom,
P.Nordlund.
A Protein Carboxylate Coordinated Oxo-Centered Tri-Nuclear Iron Complex with Possible Implications For Ferritin Mineralization Febs Lett. V. 567 179 2004.
ISSN: ISSN 0014-5793
PubMed: 15178319
DOI: 10.1016/J.FEBSLET.2004.04.068
Page generated: Sat Aug 3 12:36:17 2024
ISSN: ISSN 0014-5793
PubMed: 15178319
DOI: 10.1016/J.FEBSLET.2004.04.068
Last articles
Cl in 2Y2ICl in 2Y1X
Cl in 2Y1G
Cl in 2Y1F
Cl in 2Y05
Cl in 2Y1K
Cl in 2XZR
Cl in 2Y08
Cl in 2Y1D
Cl in 2XZ5