Iron »
PDB 1uwm-1vme »
1v55 »
Iron in PDB 1v55: Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
Enzymatic activity of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
All present enzymatic activity of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State:
1.9.3.1;
1.9.3.1;
Protein crystallography data
The structure of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State, PDB code: 1v55
was solved by
T.Tsukihara,
K.Shimokata,
Y.Katayama,
H.Shimada,
K.Muramoto,
H.Aoyama,
M.Mochizuki,
K.Shinzawa-Itoh,
E.Yamashita,
M.Yao,
Y.Ishimura,
S.Yoshikawa,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 183.060, 206.584, 178.298, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.3 / 23 |
Other elements in 1v55:
The structure of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
| Zinc | (Zn) | 2 atoms |
| Copper | (Cu) | 6 atoms |
| Sodium | (Na) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
(pdb code 1v55). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State, PDB code: 1v55:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State, PDB code: 1v55:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 1v55
Go back to
Iron binding site 1 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
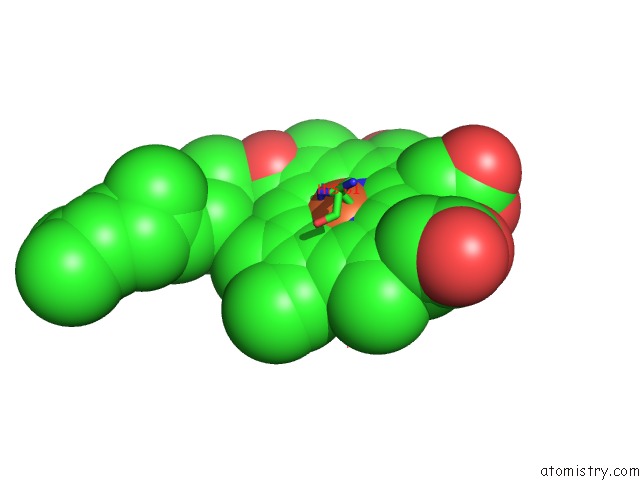
Mono view
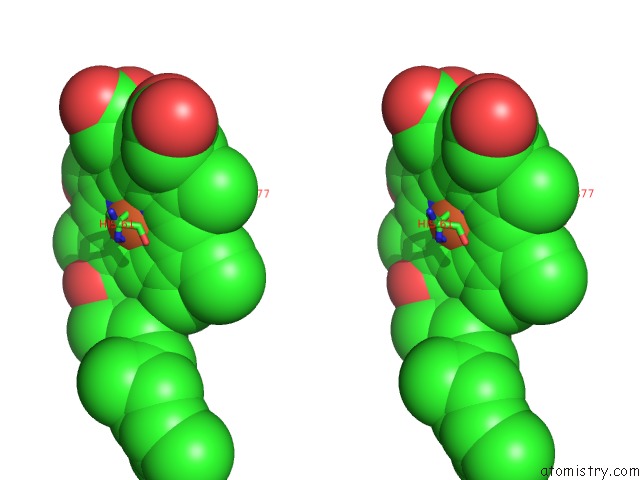
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State within 5.0Å range:
|
Iron binding site 2 out of 4 in 1v55
Go back to
Iron binding site 2 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
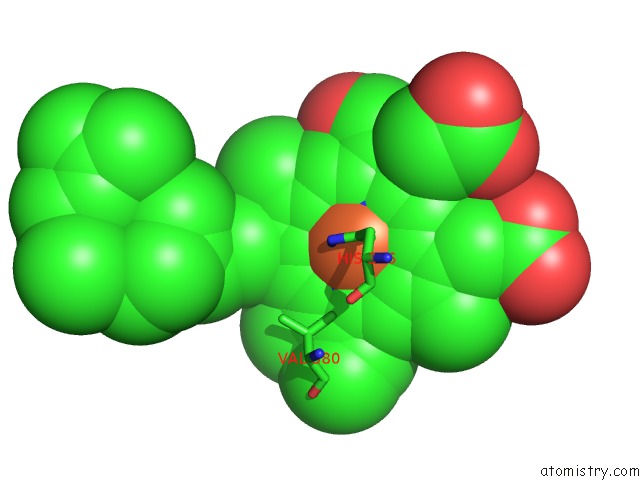
Mono view
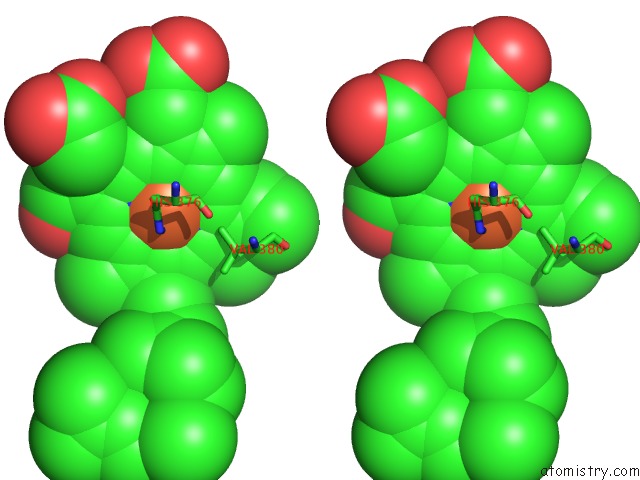
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State within 5.0Å range:
|
Iron binding site 3 out of 4 in 1v55
Go back to
Iron binding site 3 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State within 5.0Å range:
|
Iron binding site 4 out of 4 in 1v55
Go back to
Iron binding site 4 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Reduced State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Bovine Heart Cytochrome C Oxidase at the Fully Reduced State within 5.0Å range:
|
Reference:
T.Tsukihara,
K.Shimokata,
Y.Katayama,
H.Shimada,
K.Muramoto,
H.Aoyama,
M.Mochizuki,
K.Shinzawa-Itoh,
E.Yamashita,
M.Yao,
Y.Ishimura,
S.Yoshikawa.
The Low-Spin Heme of Cytochrome C Oxidase As the Driving Element of the Proton-Pumping Process. Proc.Natl.Acad.Sci.Usa V. 100 15304 2003.
ISSN: ISSN 0027-8424
PubMed: 14673090
DOI: 10.1073/PNAS.2635097100
Page generated: Sat Aug 3 16:08:41 2024
ISSN: ISSN 0027-8424
PubMed: 14673090
DOI: 10.1073/PNAS.2635097100
Last articles
F in 7LZUF in 7LZW
F in 7LY8
F in 7LWG
F in 7LZV
F in 7LZF
F in 7LZD
F in 7LZA
F in 7LVX
F in 7LUN