Iron »
PDB 1uwm-1vme »
1v8x »
Iron in PDB 1v8x: Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
Enzymatic activity of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
All present enzymatic activity of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae:
1.14.99.3;
1.14.99.3;
Protein crystallography data
The structure of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae, PDB code: 1v8x
was solved by
M.Unno,
T.Matsui,
G.C.Chu,
M.Couture,
T.Yoshida,
D.L.Rousseau,
J.S.Olson,
M.Ikeda-Saito,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.85 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.003, 62.969, 107.490, 90.00, 100.96, 90.00 |
| R / Rfree (%) | 15.3 / 19.3 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
(pdb code 1v8x). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 3 binding sites of Iron where determined in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae, PDB code: 1v8x:
Jump to Iron binding site number: 1; 2; 3;
In total 3 binding sites of Iron where determined in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae, PDB code: 1v8x:
Jump to Iron binding site number: 1; 2; 3;
Iron binding site 1 out of 3 in 1v8x
Go back to
Iron binding site 1 out
of 3 in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
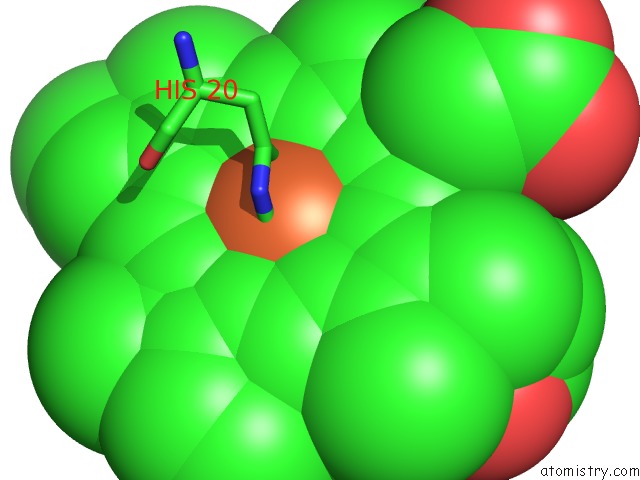
Mono view
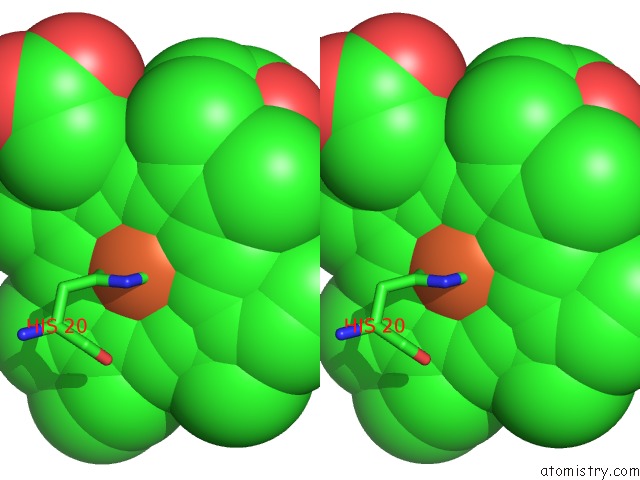
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae within 5.0Å range:
|
Iron binding site 2 out of 3 in 1v8x
Go back to
Iron binding site 2 out
of 3 in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
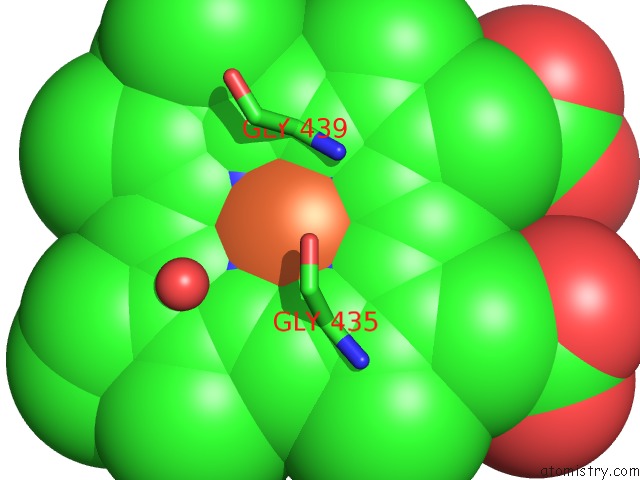
Mono view
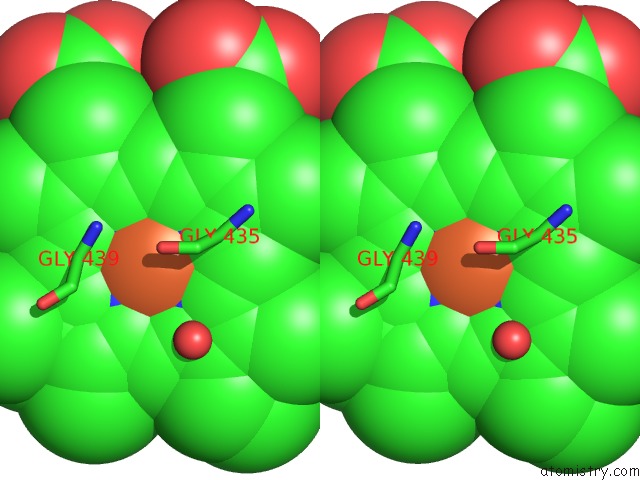
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae within 5.0Å range:
|
Iron binding site 3 out of 3 in 1v8x
Go back to
Iron binding site 3 out
of 3 in the Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae
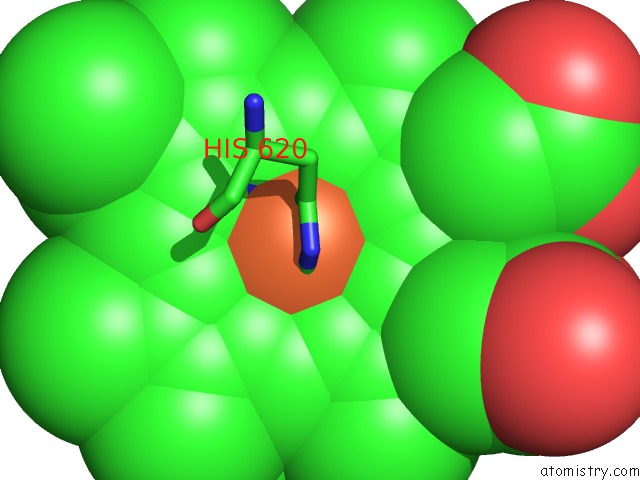
Mono view
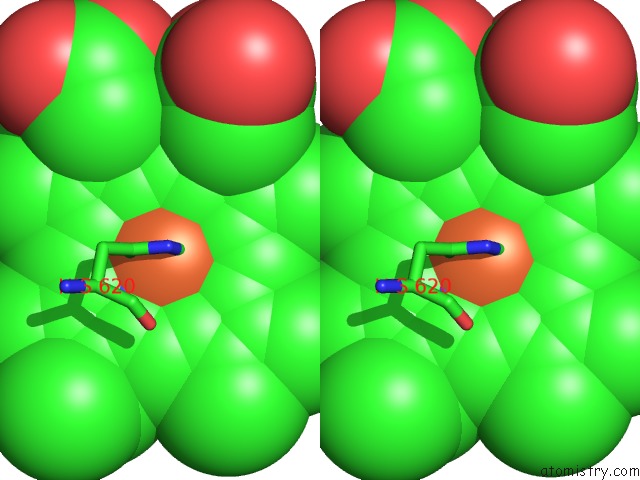
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae within 5.0Å range:
|
Reference:
M.Unno,
T.Matsui,
G.C.Chu,
M.Couture,
T.Yoshida,
D.L.Rousseau,
J.S.Olson,
M.Ikeda-Saito.
Crystal Structure of the Dioxygen-Bound Heme Oxygenase From Corynebacterium Diphtheriae: Implications For Heme Oxygenase Function. J.Biol.Chem. V. 279 21055 2004.
ISSN: ISSN 0021-9258
PubMed: 14966119
DOI: 10.1074/JBC.M400491200
Page generated: Wed Jul 16 21:36:39 2025
ISSN: ISSN 0021-9258
PubMed: 14966119
DOI: 10.1074/JBC.M400491200
Last articles
Fe in 2JIQFe in 2JIP
Fe in 2JIO
Fe in 2JI3
Fe in 2JIM
Fe in 2JH3
Fe in 2JI2
Fe in 2JI1
Fe in 2JHO
Fe in 2JE3