Iron »
PDB 1yqp-1zj9 »
1z01 »
Iron in PDB 1z01: 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
Enzymatic activity of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
All present enzymatic activity of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction:
1.14.13.61;
1.14.13.61;
Protein crystallography data
The structure of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction, PDB code: 1z01
was solved by
B.M.Martins,
T.Svetlitchnaia,
H.Dobbek,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 1.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 104.460, 166.960, 173.460, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22 / 26 |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 18;Binding sites:
The binding sites of Iron atom in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction (pdb code 1z01). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 18 binding sites of Iron where determined in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction, PDB code: 1z01:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 18 in 1z01
Go back to
Iron binding site 1 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
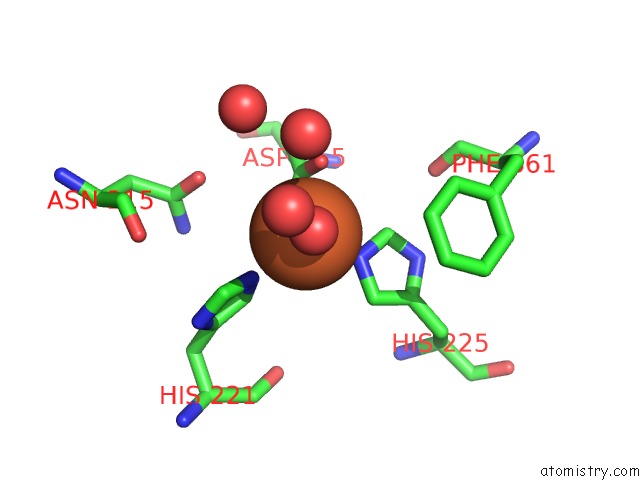
Mono view
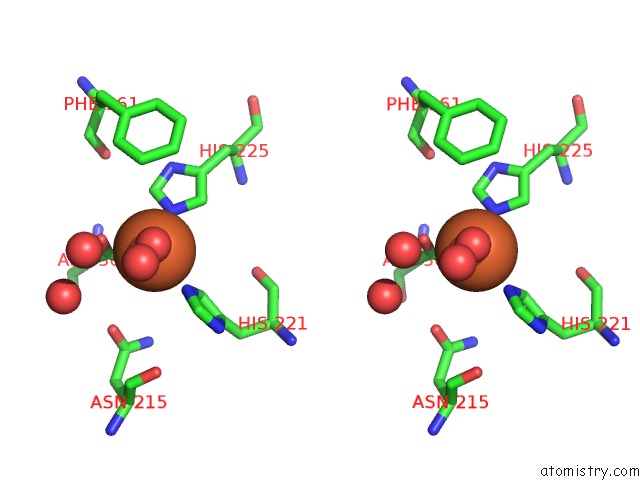
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 2 out of 18 in 1z01
Go back to
Iron binding site 2 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
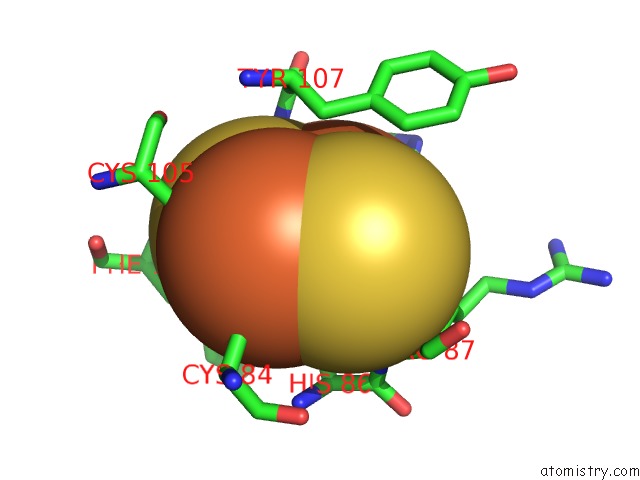
Mono view
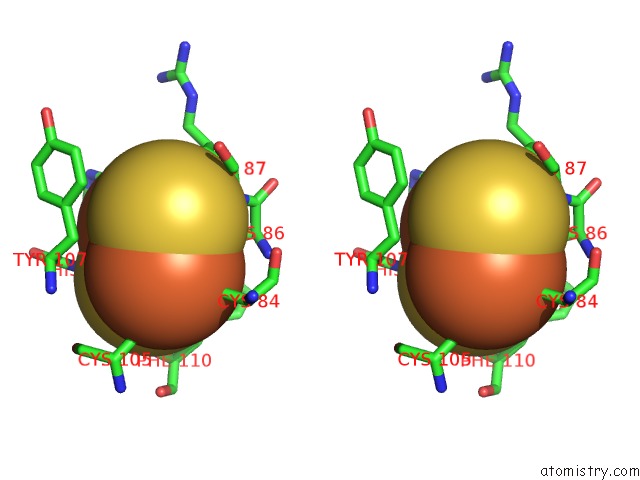
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 3 out of 18 in 1z01
Go back to
Iron binding site 3 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
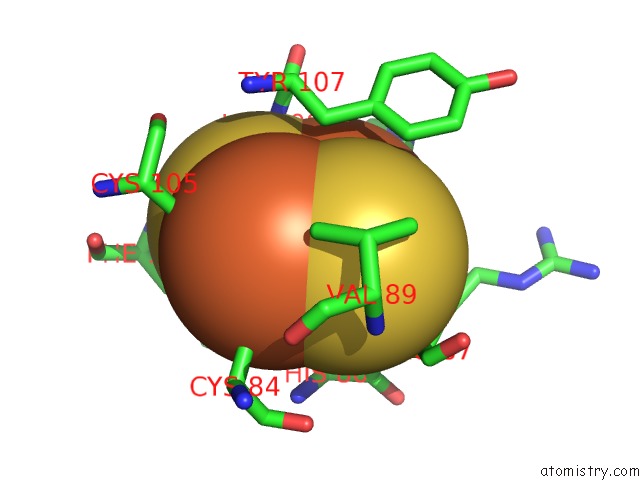
Mono view
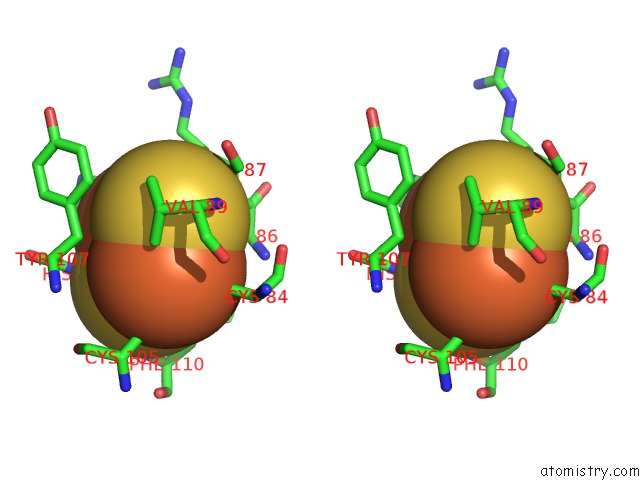
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 4 out of 18 in 1z01
Go back to
Iron binding site 4 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
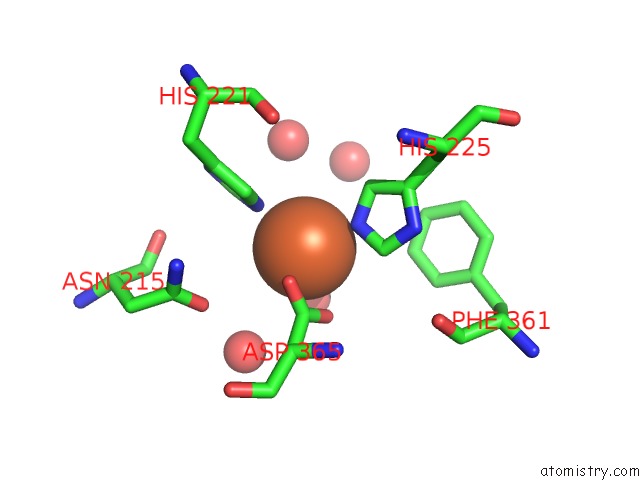
Mono view
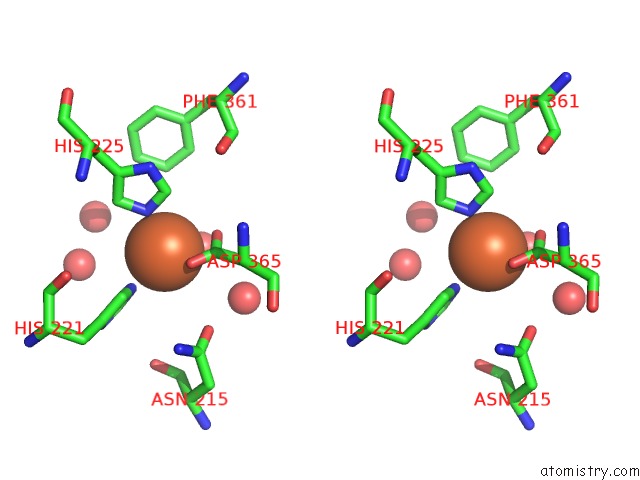
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 5 out of 18 in 1z01
Go back to
Iron binding site 5 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
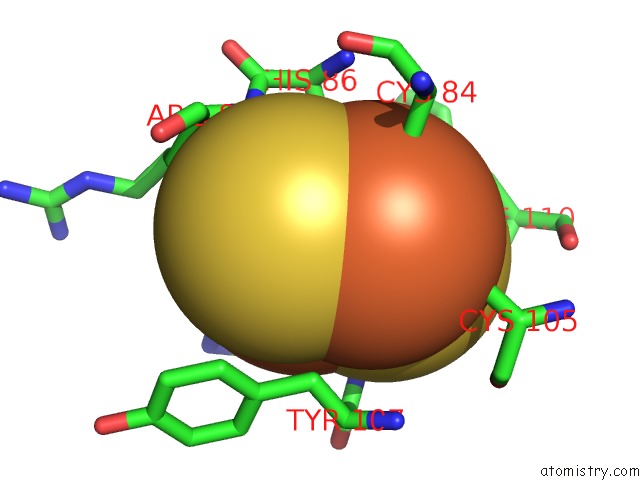
Mono view
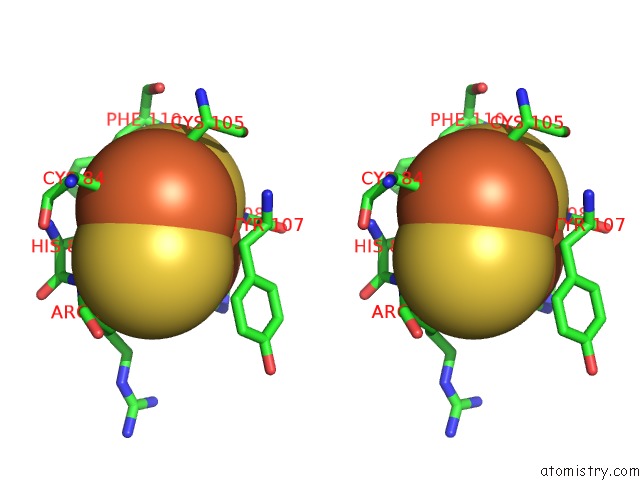
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 6 out of 18 in 1z01
Go back to
Iron binding site 6 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
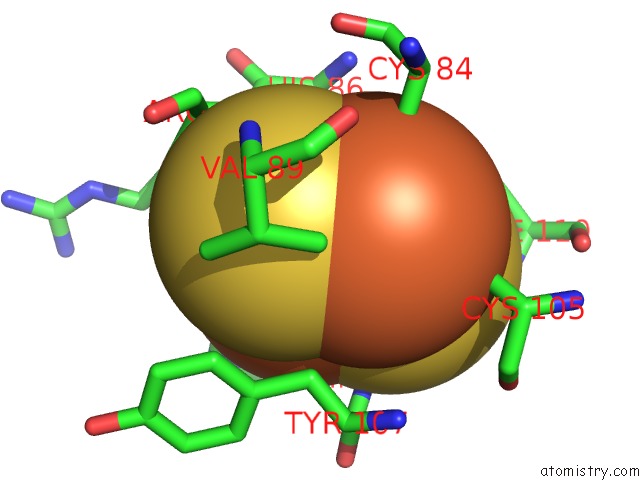
Mono view
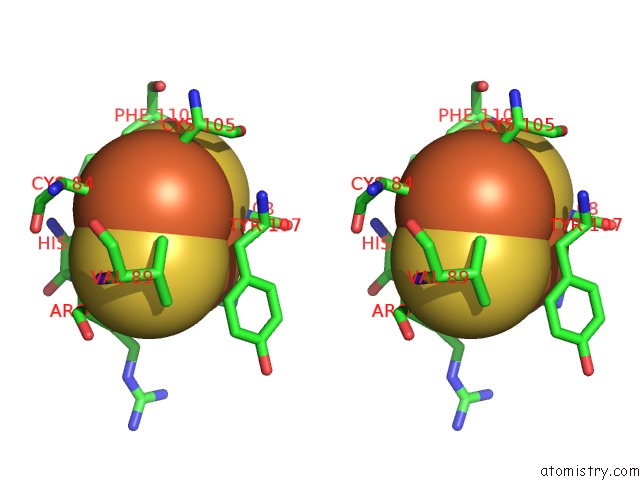
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 7 out of 18 in 1z01
Go back to
Iron binding site 7 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
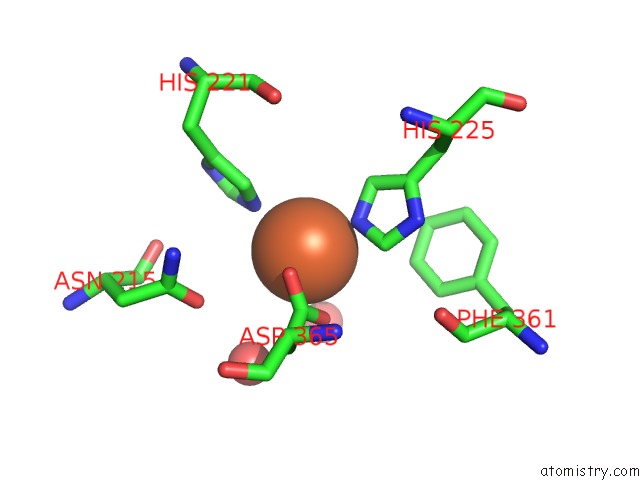
Mono view
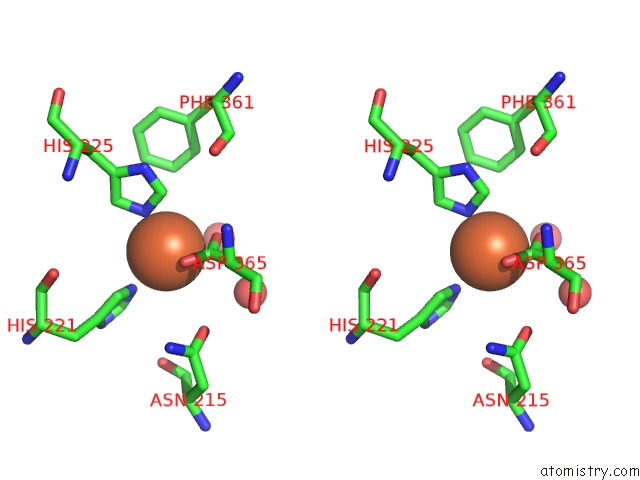
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Iron binding site 8 out of 18 in 1z01
Go back to
Iron binding site 8 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
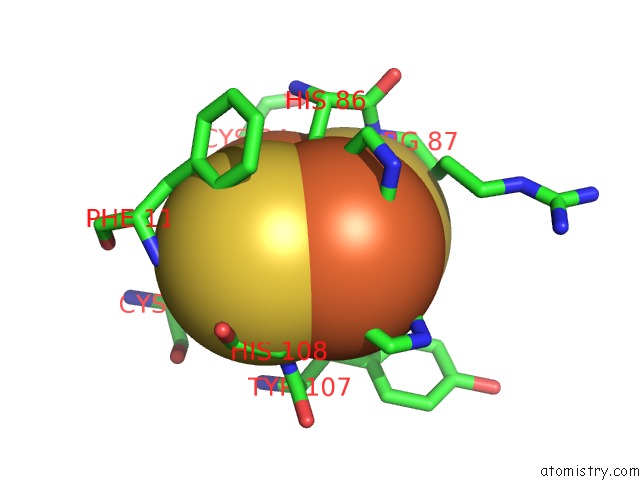
Mono view
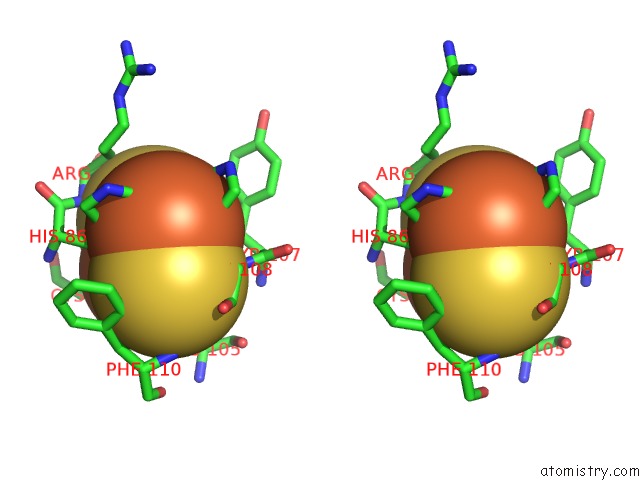
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
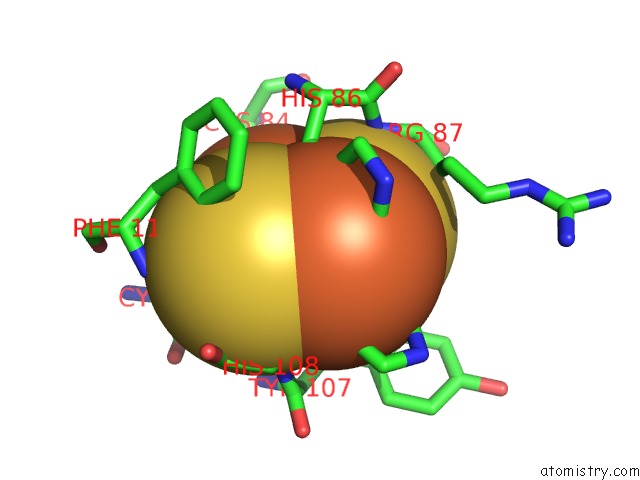
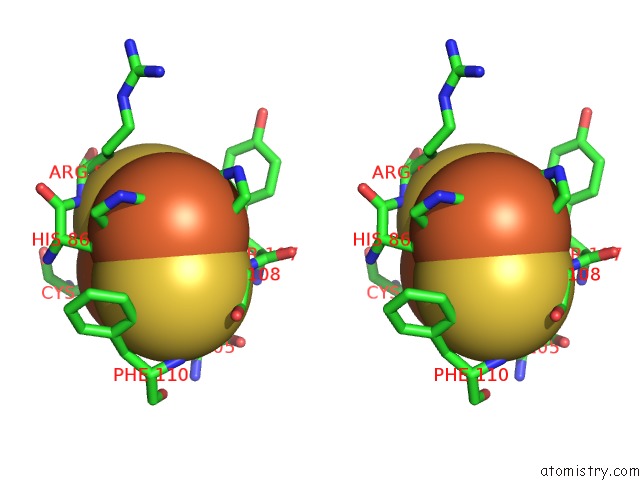
Iron binding site 9 out of 18 in 1z01
Go back to
Iron binding site 9 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
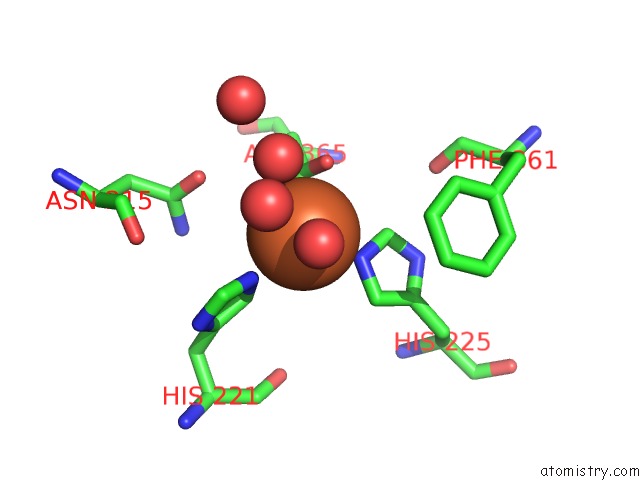
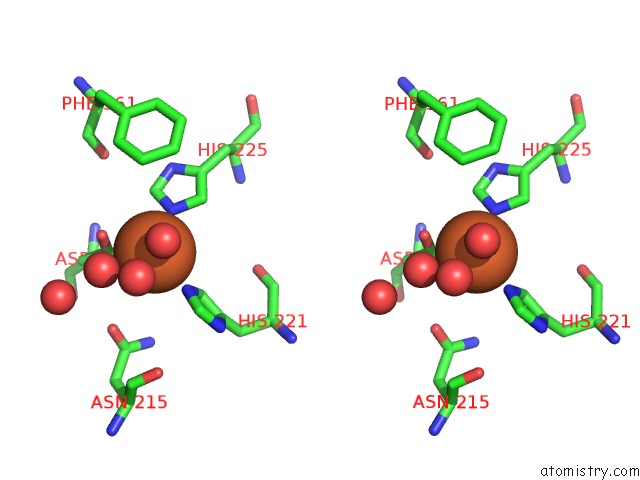
Iron binding site 10 out of 18 in 1z01
Go back to
Iron binding site 10 out
of 18 in the 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of 2-Oxoquinoline 8-Monooxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction within 5.0Å range:
|
Reference:
B.M.Martins,
T.Svetlitchnaia,
H.Dobbek.
2-Oxoquinoline 8-Monooxygenase Oxygenase Component: Active Site Modulation By Rieske-[2FE-2S] Center Oxidation/Reduction Structure V. 13 817 2005.
ISSN: ISSN 0969-2126
PubMed: 15893671
DOI: 10.1016/J.STR.2005.03.008
Page generated: Wed Jul 16 22:59:08 2025
ISSN: ISSN 0969-2126
PubMed: 15893671
DOI: 10.1016/J.STR.2005.03.008
Last articles
Fe in 2YAJFe in 2YCL
Fe in 2YDE
Fe in 2Y8N
Fe in 2YCG
Fe in 2YCC
Fe in 2YCA
Fe in 2YC0
Fe in 2YAX
Fe in 2YAW