Iron »
PDB 2d3y-2e1q »
2dyr »
Iron in PDB 2dyr: Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
Enzymatic activity of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
All present enzymatic activity of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State:
1.9.3.1;
1.9.3.1;
Protein crystallography data
The structure of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State, PDB code: 2dyr
was solved by
K.Shinzawa-Itoh,
H.Aoyama,
K.Muramoto,
T.Kurauchi,
T.Mizushima,
E.Yamashita,
T.Tsukihara,
S.Yoshikawa,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 182.590, 205.140, 178.250, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.2 / 22.7 |
Other elements in 2dyr:
The structure of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
| Zinc | (Zn) | 2 atoms |
| Copper | (Cu) | 6 atoms |
| Sodium | (Na) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
(pdb code 2dyr). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State, PDB code: 2dyr:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State, PDB code: 2dyr:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 2dyr
Go back to
Iron binding site 1 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
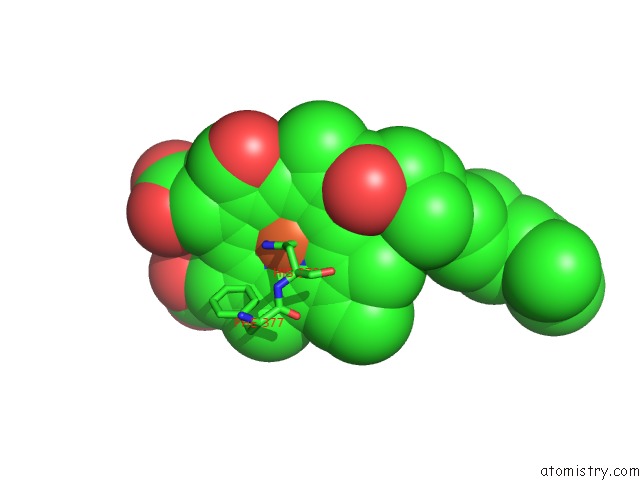
Mono view
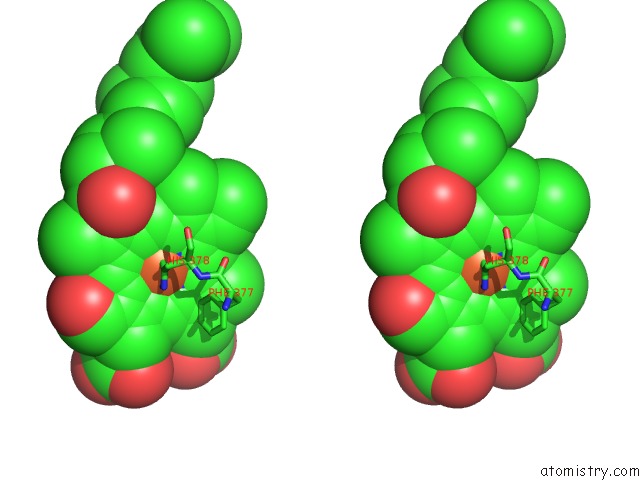
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State within 5.0Å range:
|
Iron binding site 2 out of 4 in 2dyr
Go back to
Iron binding site 2 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
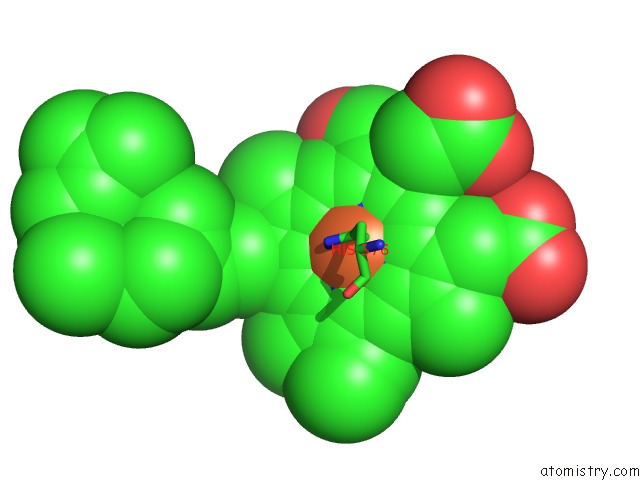
Mono view
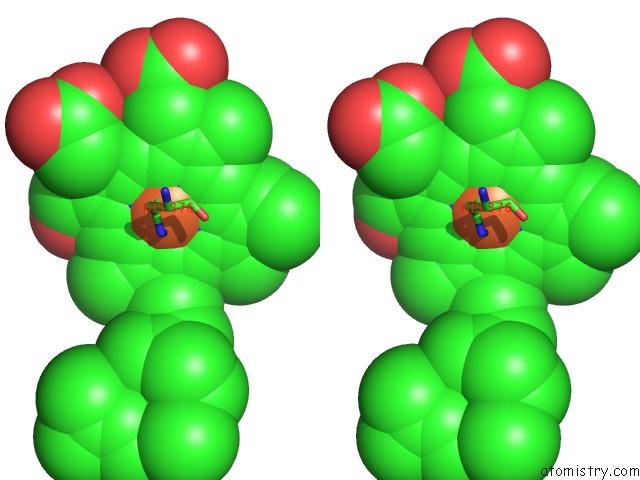
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State within 5.0Å range:
|
Iron binding site 3 out of 4 in 2dyr
Go back to
Iron binding site 3 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State within 5.0Å range:
|
Iron binding site 4 out of 4 in 2dyr
Go back to
Iron binding site 4 out
of 4 in the Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Bovine Heart Cytochrome C Oxidase at the Fully Oxidized State within 5.0Å range:
|
Reference:
K.Shinzawa-Itoh,
H.Aoyama,
K.Muramoto,
H.Terada,
T.Kurauchi,
Y.Tadehara,
A.Yamasaki,
T.Sugimura,
S.Kurono,
K.Tsujimoto,
T.Mizushima,
E.Yamashita,
T.Tsukihara,
S.Yoshikawa.
Structures and Physiological Roles of 13 Integral Lipids of Bovine Heart Cytochrome C Oxidase Embo J. V. 26 1713 2007.
ISSN: ISSN 0261-4189
PubMed: 17332748
DOI: 10.1038/SJ.EMBOJ.7601618
Page generated: Sat Aug 3 20:52:55 2024
ISSN: ISSN 0261-4189
PubMed: 17332748
DOI: 10.1038/SJ.EMBOJ.7601618
Last articles
F in 4J52F in 4J1H
F in 4J1E
F in 4J1F
F in 4J1C
F in 4J17
F in 4J0Z
F in 4J0V
F in 4J0Y
F in 4J14