Iron »
PDB 2nwf-2oof »
2ohj »
Iron in PDB 2ohj: Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
Protein crystallography data
The structure of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State, PDB code: 2ohj
was solved by
H.Seedorf,
E.Warkentin,
U.Ermler,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.11 / 2.26 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.700, 88.700, 450.400, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.6 / 23.4 |
Other elements in 2ohj:
The structure of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State also contains other interesting chemical elements:
| Chlorine | (Cl) | 7 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Iron atom in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State (pdb code 2ohj). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 12 binding sites of Iron where determined in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State, PDB code: 2ohj:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 12 in 2ohj
Go back to
Iron binding site 1 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
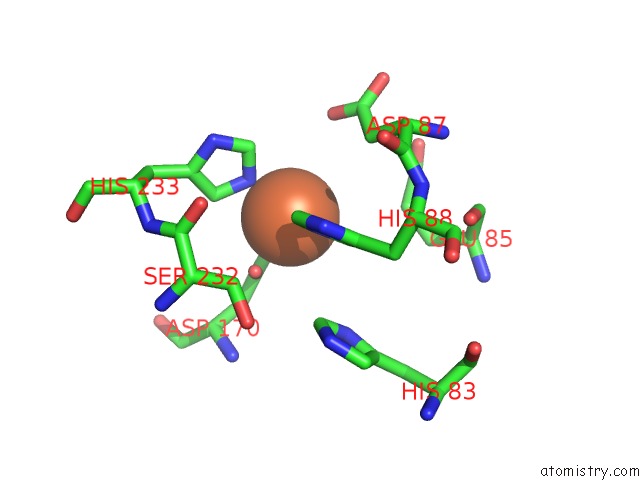
Mono view
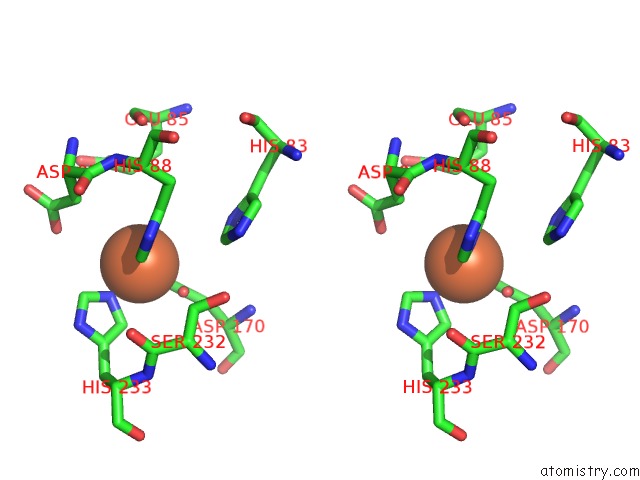
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Iron binding site 2 out of 12 in 2ohj
Go back to
Iron binding site 2 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
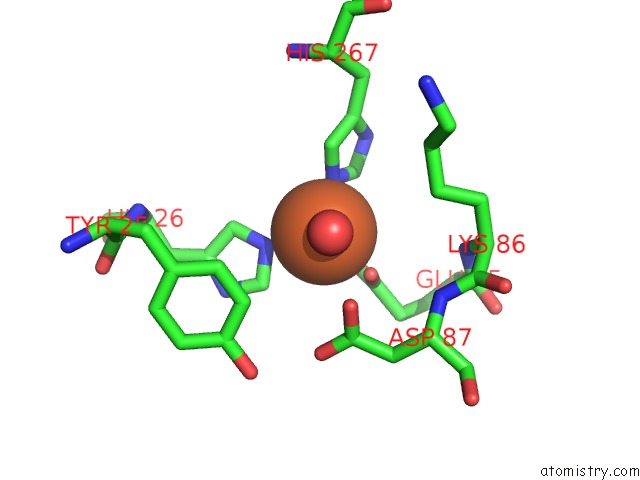
Mono view
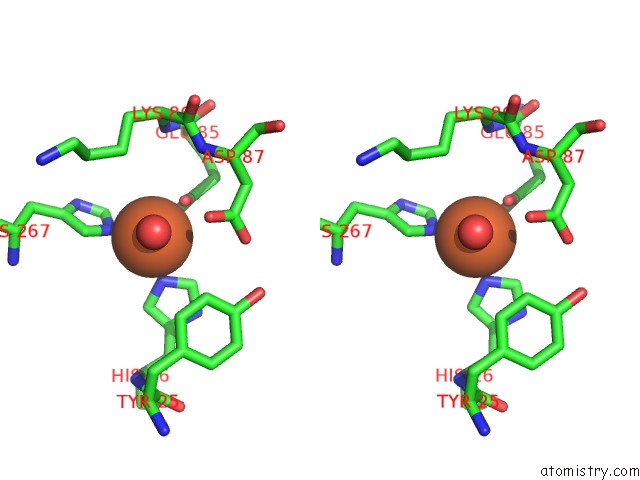
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
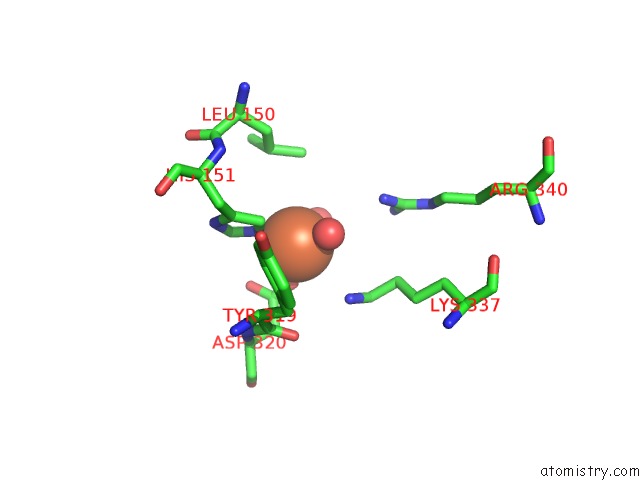
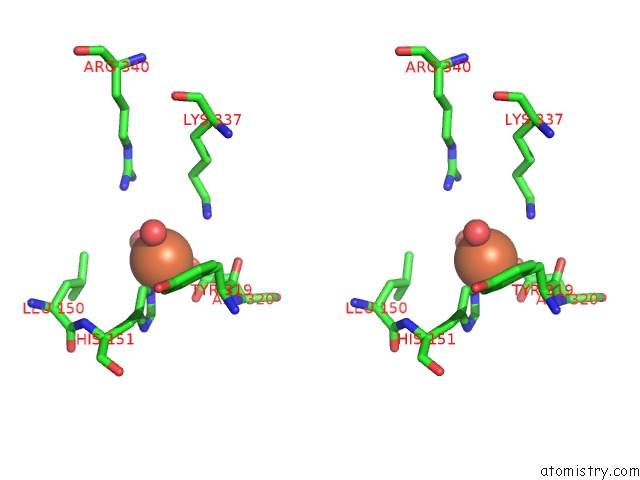
Iron binding site 3 out of 12 in 2ohj
Go back to
Iron binding site 3 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
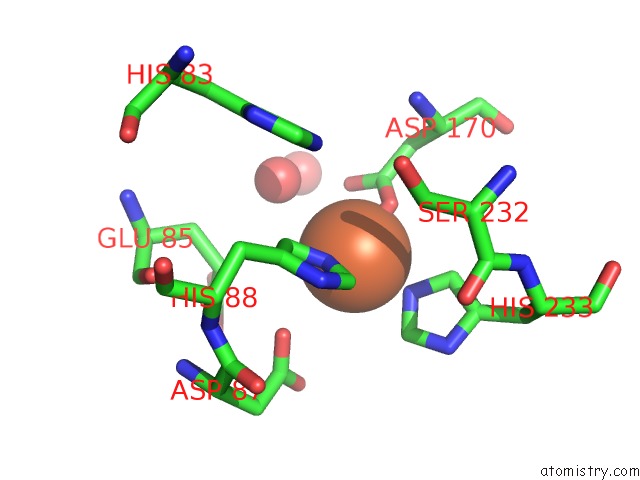
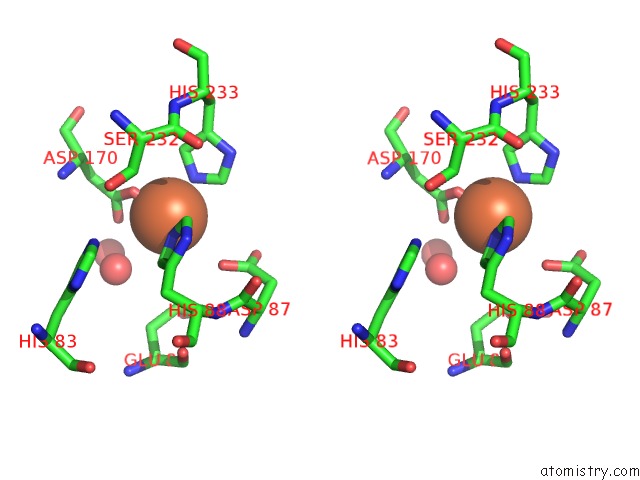
Iron binding site 4 out of 12 in 2ohj
Go back to
Iron binding site 4 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Iron binding site 5 out of 12 in 2ohj
Go back to
Iron binding site 5 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
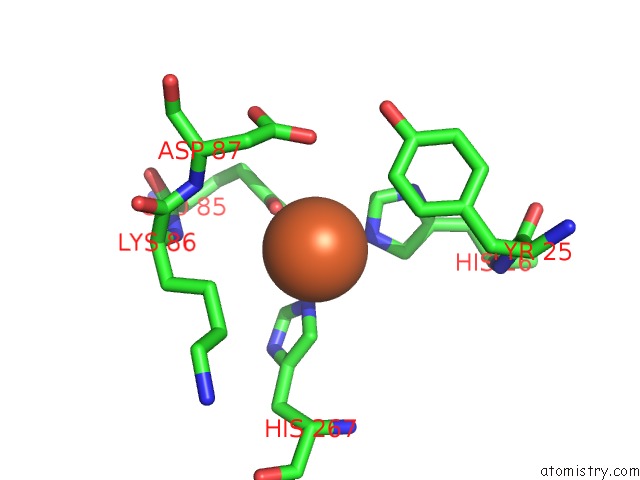
Mono view
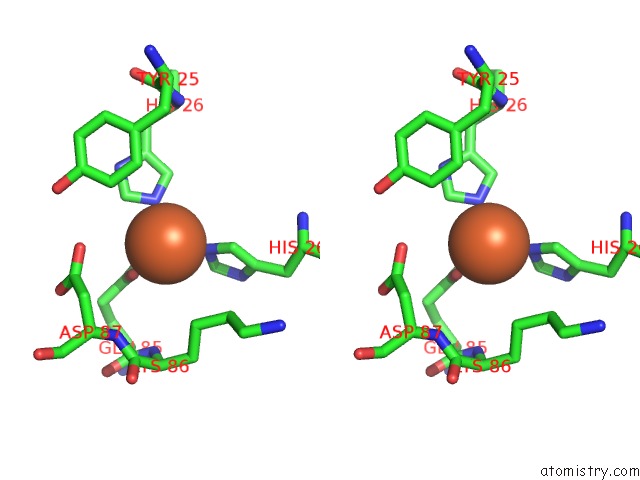
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Iron binding site 6 out of 12 in 2ohj
Go back to
Iron binding site 6 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
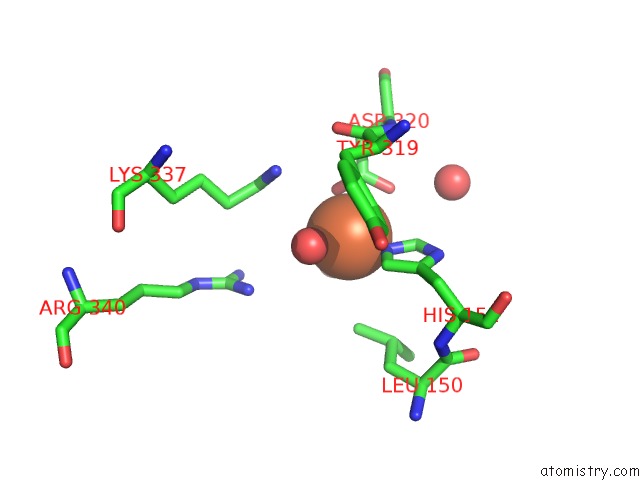
Mono view
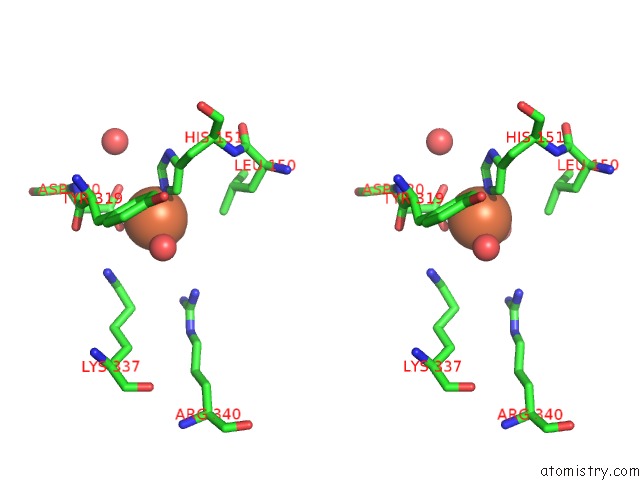
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
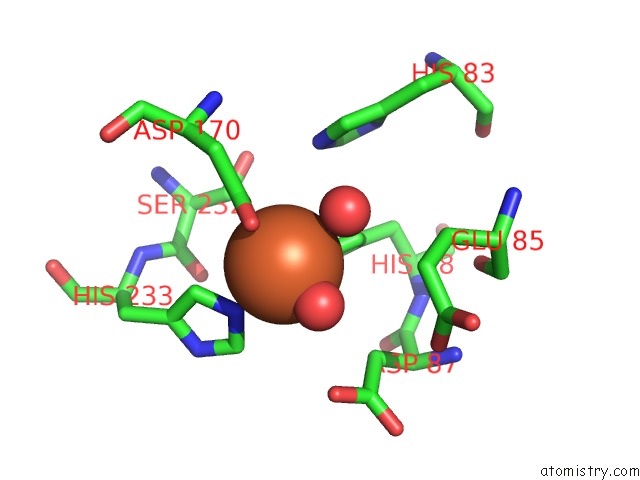
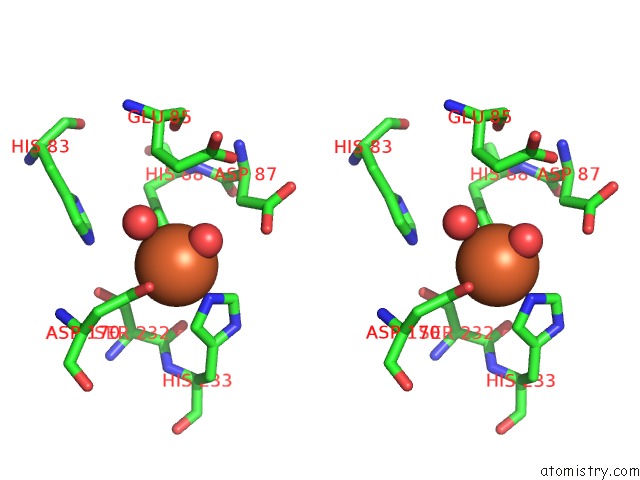
Iron binding site 7 out of 12 in 2ohj
Go back to
Iron binding site 7 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
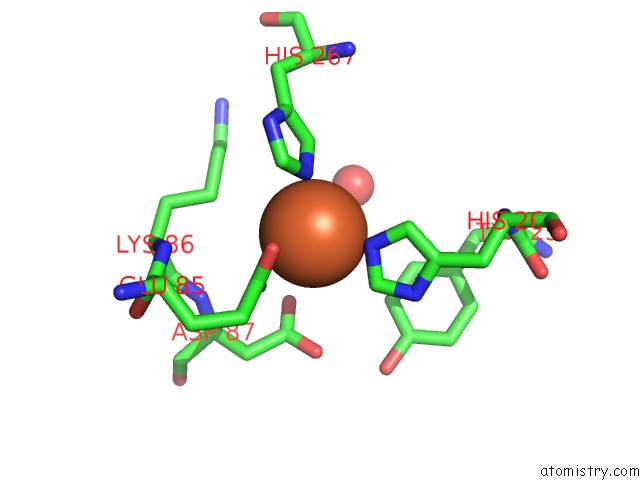
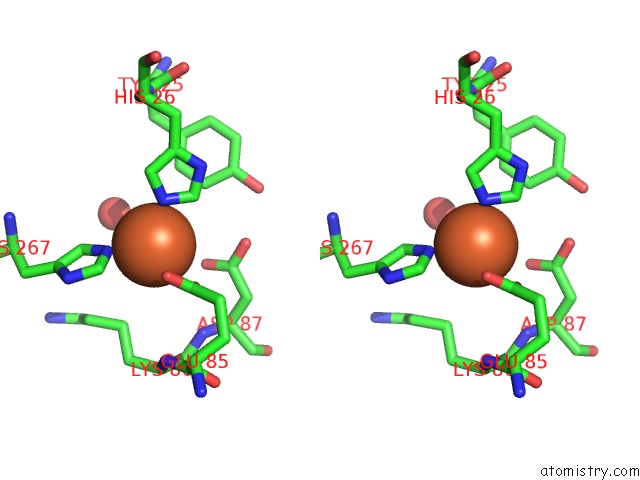
Iron binding site 8 out of 12 in 2ohj
Go back to
Iron binding site 8 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Iron binding site 9 out of 12 in 2ohj
Go back to
Iron binding site 9 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
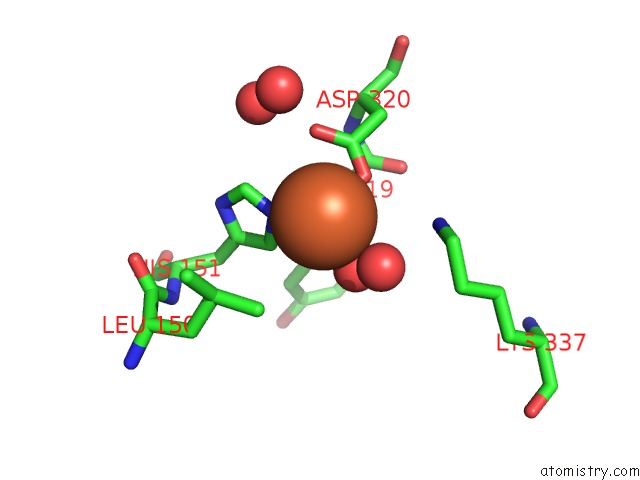
Mono view
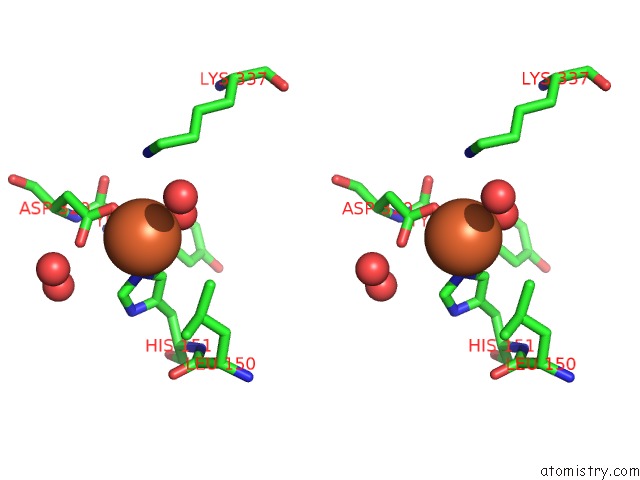
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Iron binding site 10 out of 12 in 2ohj
Go back to
Iron binding site 10 out
of 12 in the Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State
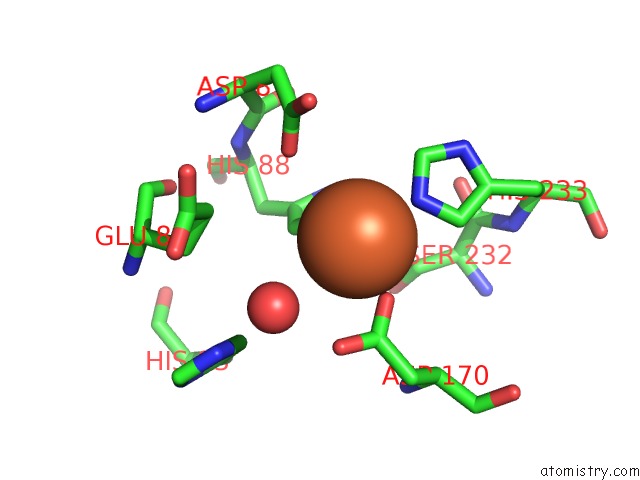
Mono view
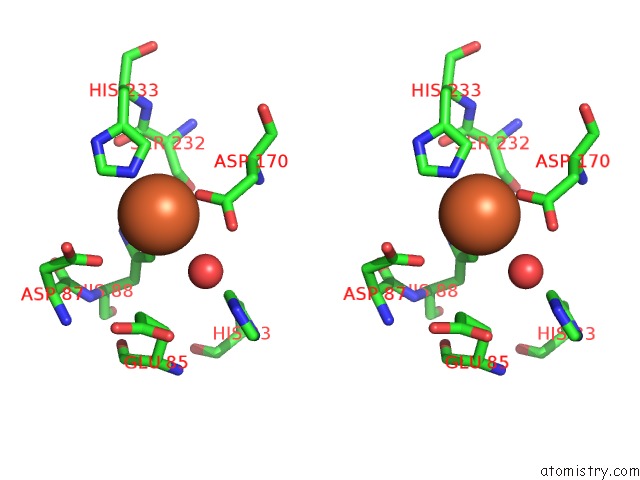
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Crystal Structure of Coenzyme F420H2 Oxidase (Fpra), A Diiron Flavoprotein, Inactive Oxidized State within 5.0Å range:
|
Reference:
H.Seedorf,
C.H.Hagemeier,
S.Shima,
R.K.Thauer,
E.Warkentin,
U.Ermler.
Structure of Coenzyme F420H2 Oxidase (Fpra), A Di-Iron Flavoprotein From Methanogenic Archaea Catalyzing the Reduction of O2 to H2O. Febs J. V. 274 1588 2007.
ISSN: ISSN 1742-464X
PubMed: 17480207
DOI: 10.1111/J.1742-4658.2007.05706.X
Page generated: Sun Aug 4 00:59:17 2024
ISSN: ISSN 1742-464X
PubMed: 17480207
DOI: 10.1111/J.1742-4658.2007.05706.X
Last articles
Cl in 5SHMCl in 5SHG
Cl in 5SGZ
Cl in 5SGY
Cl in 5SGR
Cl in 5SGB
Cl in 5SGG
Cl in 5SFP
Cl in 5SFG
Cl in 5SF7