Iron »
PDB 2pgh-2q9u »
2pia »
Iron in PDB 2pia: Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S]
Protein crystallography data
The structure of Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S], PDB code: 2pia
was solved by
C.C.Correll,
C.J.Batie,
D.P.Ballou,
M.L.Ludwig,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 2.00 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.400, 114.400, 77.800, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.6 / n/a |
Iron Binding Sites:
The binding sites of Iron atom in the Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S]
(pdb code 2pia). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S], PDB code: 2pia:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S], PDB code: 2pia:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 2pia
Go back to
Iron binding site 1 out
of 2 in the Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S]
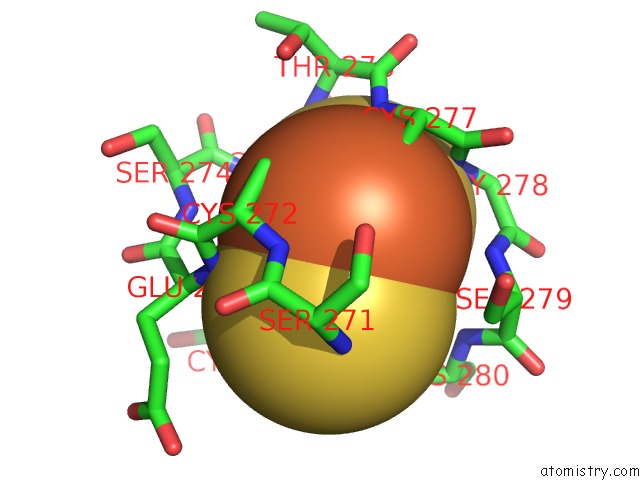
Mono view
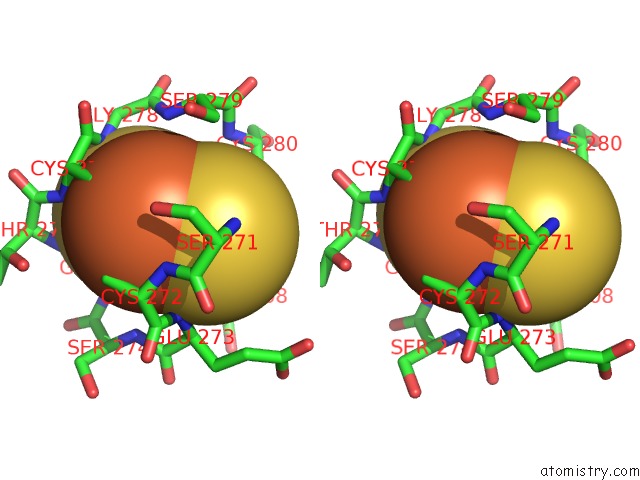
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S] within 5.0Å range:
|
Iron binding site 2 out of 2 in 2pia
Go back to
Iron binding site 2 out
of 2 in the Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S]
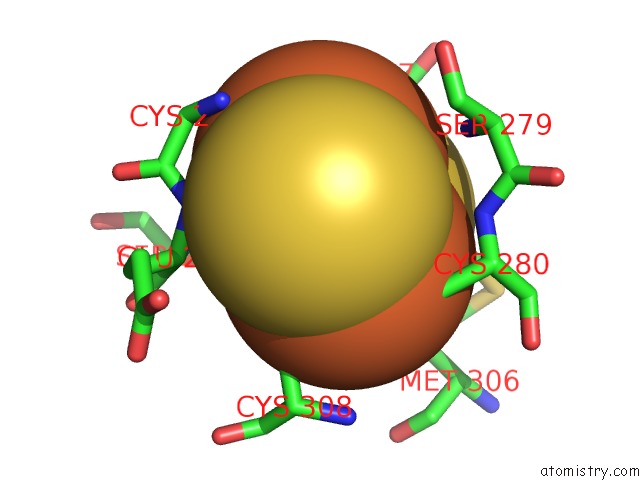
Mono view
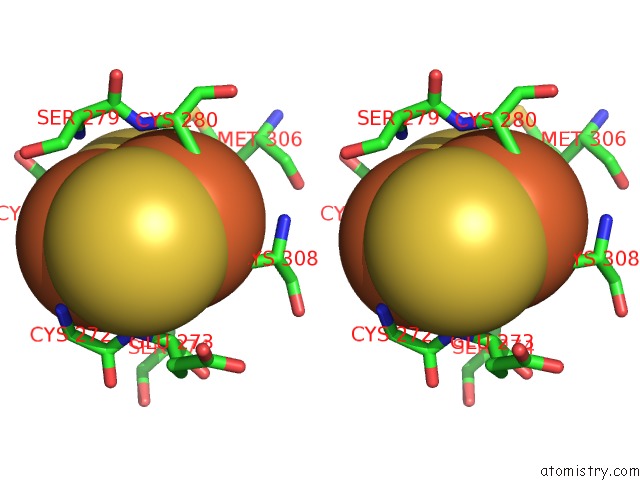
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S] within 5.0Å range:
|
Reference:
C.C.Correll,
C.J.Batie,
D.P.Ballou,
M.L.Ludwig.
Phthalate Dioxygenase Reductase: A Modular Structure For Electron Transfer From Pyridine Nucleotides to [2FE-2S]. Science V. 258 1604 1992.
ISSN: ISSN 0036-8075
PubMed: 1280857
Page generated: Thu Jul 17 03:28:25 2025
ISSN: ISSN 0036-8075
PubMed: 1280857
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO