Iron »
PDB 2rfc-2v1i »
2toh »
Iron in PDB 2toh: Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat
Enzymatic activity of Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat
All present enzymatic activity of Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat:
1.14.16.2;
1.14.16.2;
Protein crystallography data
The structure of Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat, PDB code: 2toh
was solved by
K.E.Goodwill,
C.Sabatier,
R.C.Stevens,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 2.30 |
| Space group | F 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 189.464, 148.595, 56.986, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.7 / 27.6 |
Other elements in 2toh:
The structure of Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat
(pdb code 2toh). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat, PDB code: 2toh:
In total only one binding site of Iron was determined in the Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat, PDB code: 2toh:
Iron binding site 1 out of 1 in 2toh
Go back to
Iron binding site 1 out
of 1 in the Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat
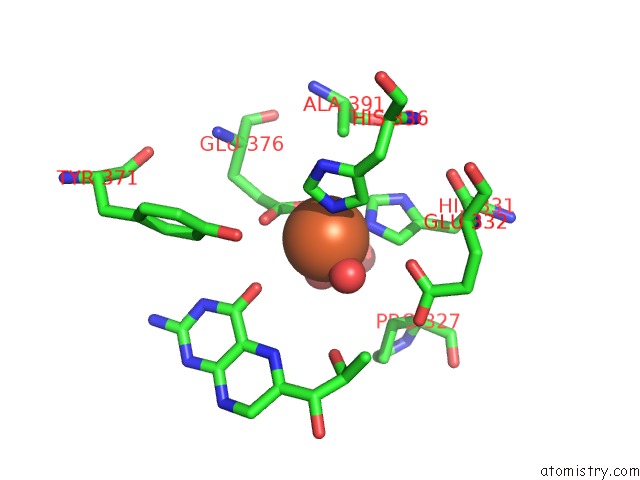
Mono view
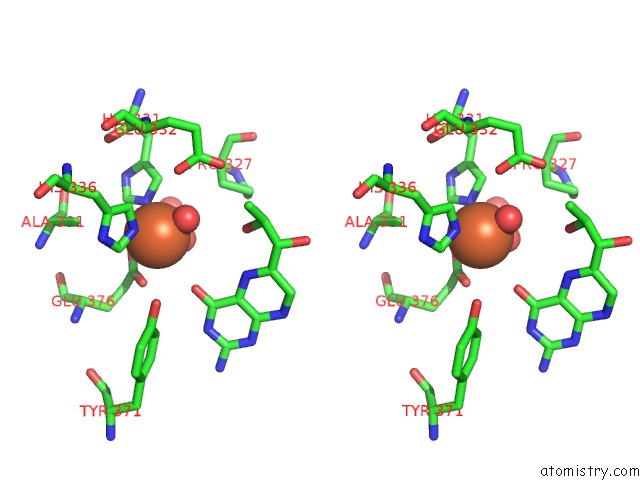
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Tyrosine Hydroxylase Catalytic and Tetramerization Domains From Rat within 5.0Å range:
|
Reference:
K.E.Goodwill,
C.Sabatier,
R.C.Stevens.
Crystal Structure of Tyrosine Hydroxylase with Bound Cofactor Analogue and Iron at 2.3 A Resolution: Self-Hydroxylation of PHE300 and the Pterin-Binding Site. Biochemistry V. 37 13437 1998.
ISSN: ISSN 0006-2960
PubMed: 9753429
DOI: 10.1021/BI981462G
Page generated: Thu Jul 17 04:02:41 2025
ISSN: ISSN 0006-2960
PubMed: 9753429
DOI: 10.1021/BI981462G
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO