Iron »
PDB 3a0g-3ae5 »
3a4h »
Iron in PDB 3a4h: Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form)
Protein crystallography data
The structure of Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form), PDB code: 3a4h
was solved by
Y.Yasutake,
Y.Fujii,
W.K.Cheon,
A.Arisawa,
T.Tamura,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.74 / 3.06 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 63.595, 65.793, 102.264, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.7 / 27 |
Other elements in 3a4h:
The structure of Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form) also contains other interesting chemical elements:
| Calcium | (Ca) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form)
(pdb code 3a4h). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form), PDB code: 3a4h:
In total only one binding site of Iron was determined in the Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form), PDB code: 3a4h:
Iron binding site 1 out of 1 in 3a4h
Go back to
Iron binding site 1 out
of 1 in the Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form)
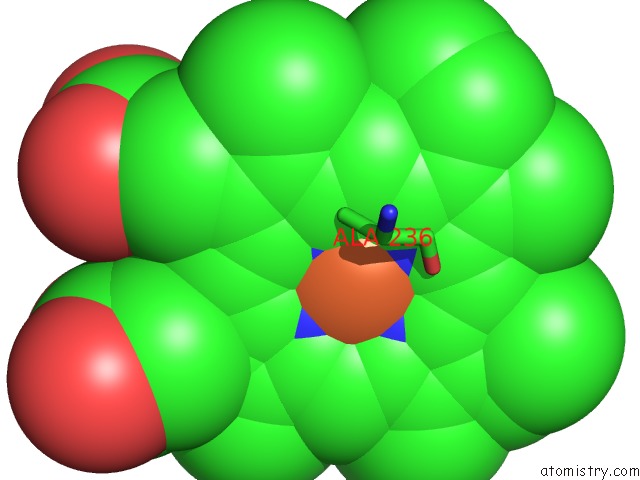
Mono view
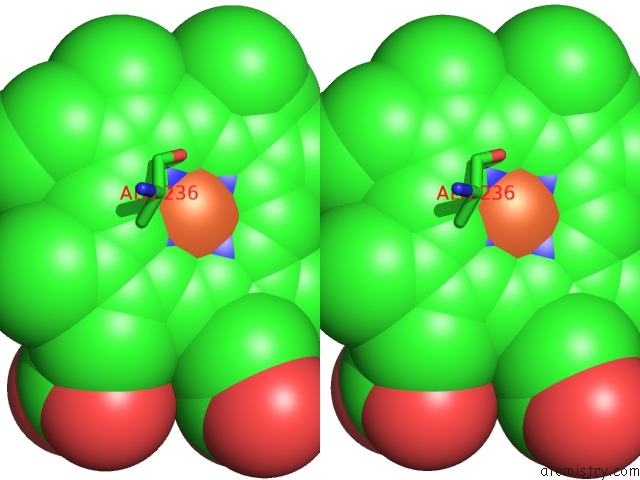
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Cytochrome P450 Vdh From Pseudonocardia Autotrophica (Orthorhombic Crystal Form) within 5.0Å range:
|
Reference:
Y.Yasutake,
Y.Fujii,
T.Nishioka,
W.K.Cheon,
A.Arisawa,
T.Tamura.
Structural Evidence For Enhancement of Sequential Vitamin D3 Hydroxylation Activities By Directed Evolution of Cytochrome P450 Vitamin D3 Hydroxylase J.Biol.Chem. V. 285 31193 2010.
ISSN: ISSN 0021-9258
PubMed: 20667833
DOI: 10.1074/JBC.M110.147009
Page generated: Mon Aug 4 23:07:02 2025
ISSN: ISSN 0021-9258
PubMed: 20667833
DOI: 10.1074/JBC.M110.147009
Last articles
Fe in 3HKPFe in 3HJS
Fe in 3HJQ
Fe in 3HHB
Fe in 3HJ8
Fe in 3HHY
Fe in 3HHX
Fe in 3HF4
Fe in 3HH8
Fe in 3HGI