Iron »
PDB 3a0g-3ae5 »
3a51 »
Iron in PDB 3a51: Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3
Protein crystallography data
The structure of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3, PDB code: 3a51
was solved by
Y.Yasutake,
Y.Fujii,
W.K.Cheon,
A.Arisawa,
T.Tamura,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.60 / 2.00 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.173, 171.800, 189.143, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.7 / 23.4 |
Other elements in 3a51:
The structure of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 also contains other interesting chemical elements:
| Calcium | (Ca) | 6 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3
(pdb code 3a51). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 5 binding sites of Iron where determined in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3, PDB code: 3a51:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Iron where determined in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3, PDB code: 3a51:
Jump to Iron binding site number: 1; 2; 3; 4; 5;
Iron binding site 1 out of 5 in 3a51
Go back to
Iron binding site 1 out
of 5 in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 within 5.0Å range:
|
Iron binding site 2 out of 5 in 3a51
Go back to
Iron binding site 2 out
of 5 in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3
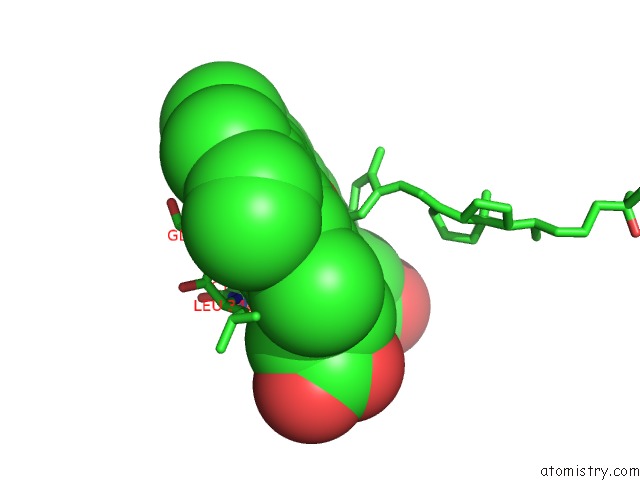
Mono view
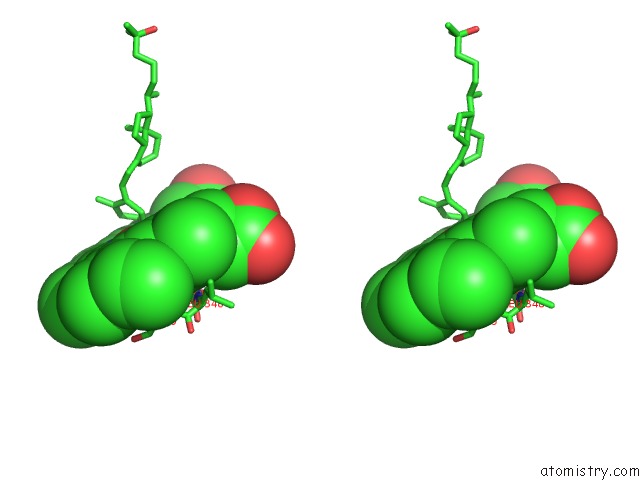
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 within 5.0Å range:
|
Iron binding site 3 out of 5 in 3a51
Go back to
Iron binding site 3 out
of 5 in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 within 5.0Å range:
|
Iron binding site 4 out of 5 in 3a51
Go back to
Iron binding site 4 out
of 5 in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 within 5.0Å range:
|
Iron binding site 5 out of 5 in 3a51
Go back to
Iron binding site 5 out
of 5 in the Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3
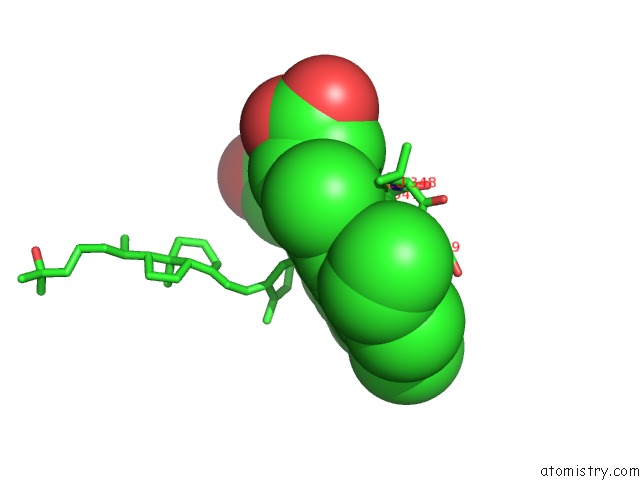
Mono view
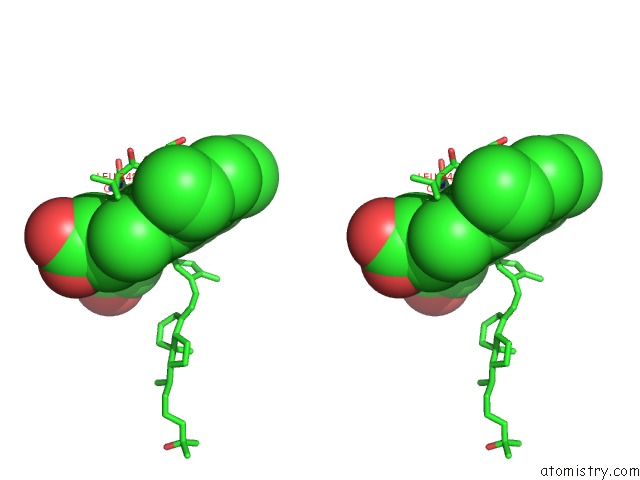
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Structure of Cytochrome P450 Vdh Mutant (Vdh-K1) Obtained By Directed Evolution with Bound 25-Hydroxyvitamin D3 within 5.0Å range:
|
Reference:
Y.Yasutake,
Y.Fujii,
T.Nishioka,
W.K.Cheon,
A.Arisawa,
T.Tamura.
Structural Evidence For Enhancement of Sequential Vitamin D3 Hydroxylation Activities By Directed Evolution of Cytochrome P450 Vitamin D3 Hydroxylase J.Biol.Chem. V. 285 31193 2010.
ISSN: ISSN 0021-9258
PubMed: 20667833
DOI: 10.1074/JBC.M110.147009
Page generated: Mon Aug 4 23:07:57 2025
ISSN: ISSN 0021-9258
PubMed: 20667833
DOI: 10.1074/JBC.M110.147009
Last articles
Fe in 3I04Fe in 3I01
Fe in 3I8R
Fe in 3I39
Fe in 3I6N
Fe in 3I63
Fe in 3I5J
Fe in 3I51
Fe in 3I4Y
Fe in 3I4V