Iron »
PDB 3na1-3nmj »
3ner »
Iron in PDB 3ner: Structure of Human Type B Cytochrome B5
Protein crystallography data
The structure of Structure of Human Type B Cytochrome B5, PDB code: 3ner
was solved by
S.Terzyan,
C.Zhang,
M.Rivera,
D.B.Benson,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.24 / 1.45 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 37.316, 40.034, 58.744, 90.00, 98.61, 90.00 |
| R / Rfree (%) | 17.7 / 21.5 |
Other elements in 3ner:
The structure of Structure of Human Type B Cytochrome B5 also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Human Type B Cytochrome B5
(pdb code 3ner). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Structure of Human Type B Cytochrome B5, PDB code: 3ner:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Structure of Human Type B Cytochrome B5, PDB code: 3ner:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 3ner
Go back to
Iron binding site 1 out
of 4 in the Structure of Human Type B Cytochrome B5
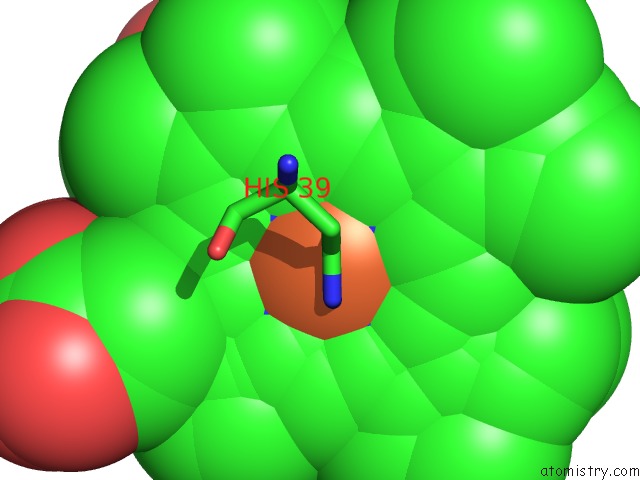
Mono view
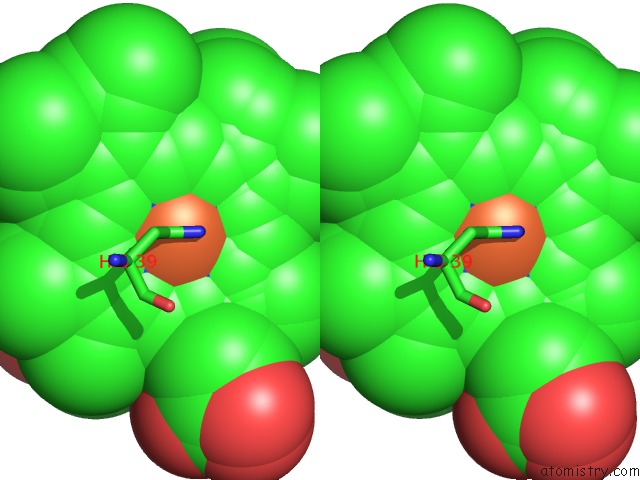
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Human Type B Cytochrome B5 within 5.0Å range:
|
Iron binding site 2 out of 4 in 3ner
Go back to
Iron binding site 2 out
of 4 in the Structure of Human Type B Cytochrome B5

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Human Type B Cytochrome B5 within 5.0Å range:
|
Iron binding site 3 out of 4 in 3ner
Go back to
Iron binding site 3 out
of 4 in the Structure of Human Type B Cytochrome B5
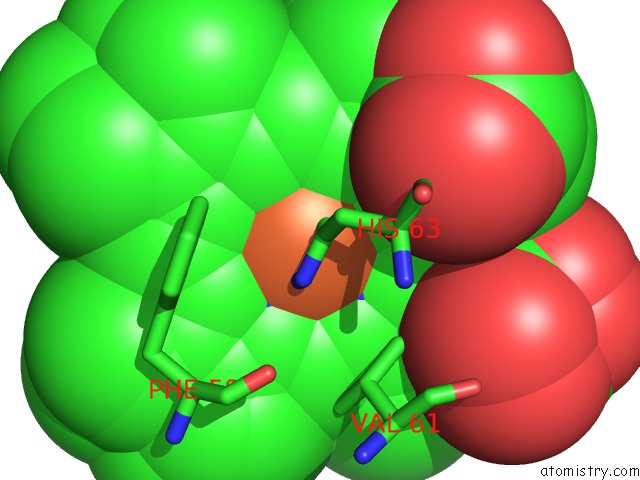
Mono view
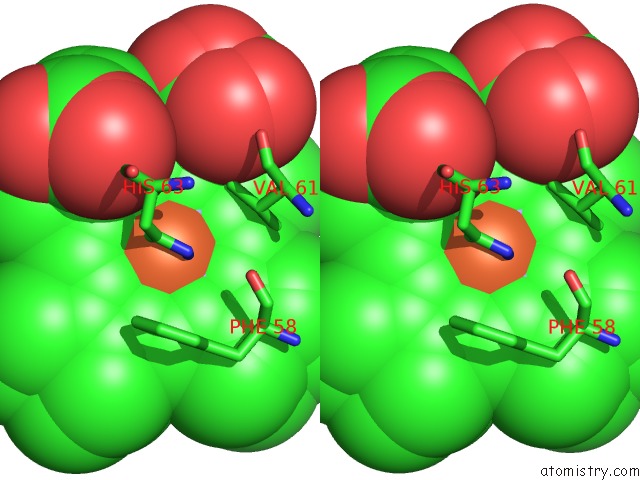
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of Human Type B Cytochrome B5 within 5.0Å range:
|
Iron binding site 4 out of 4 in 3ner
Go back to
Iron binding site 4 out
of 4 in the Structure of Human Type B Cytochrome B5

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of Human Type B Cytochrome B5 within 5.0Å range:
|
Reference:
S.Parthasarathy,
A.Altuve,
S.Terzyan,
X.Zhang,
K.Kuczera,
M.Rivera,
D.R.Benson.
Accommodating A Non-Conservative Internal Mutation By Water-Mediated Hydrogen-Bonding Between Beta-Sheet Strands: A Comparison of Human and Rat Type B (Mitochondrial) Cytochrome B5 Biochemistry V. 50 5544 2011.
ISSN: ISSN 0006-2960
PubMed: 21574570
DOI: 10.1021/BI2004729
Page generated: Sun Aug 4 16:24:56 2024
ISSN: ISSN 0006-2960
PubMed: 21574570
DOI: 10.1021/BI2004729
Last articles
Cl in 2WQ9Cl in 2WQO
Cl in 2WR6
Cl in 2WPS
Cl in 2WQ0
Cl in 2WQ3
Cl in 2WPZ
Cl in 2WPY
Cl in 2WPU
Cl in 2WPE