Iron »
PDB 3nmk-3nwv »
3nvc »
Iron in PDB 3nvc: Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate
Enzymatic activity of Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate
All present enzymatic activity of Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate:
1.13.11.4;
1.13.11.4;
Protein crystallography data
The structure of Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate, PDB code: 3nvc
was solved by
M.Ferraroni,
F.Briganti,
I.Matera,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.45 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 74.273, 86.977, 167.626, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.3 / 27.2 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate
(pdb code 3nvc). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate, PDB code: 3nvc:
In total only one binding site of Iron was determined in the Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate, PDB code: 3nvc:
Iron binding site 1 out of 1 in 3nvc
Go back to
Iron binding site 1 out
of 1 in the Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate
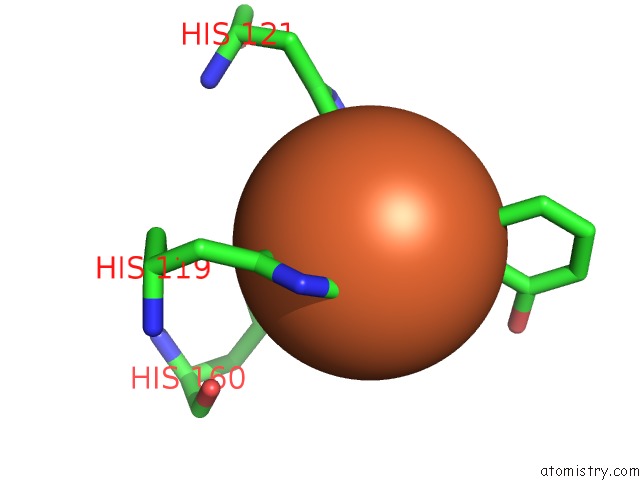
Mono view
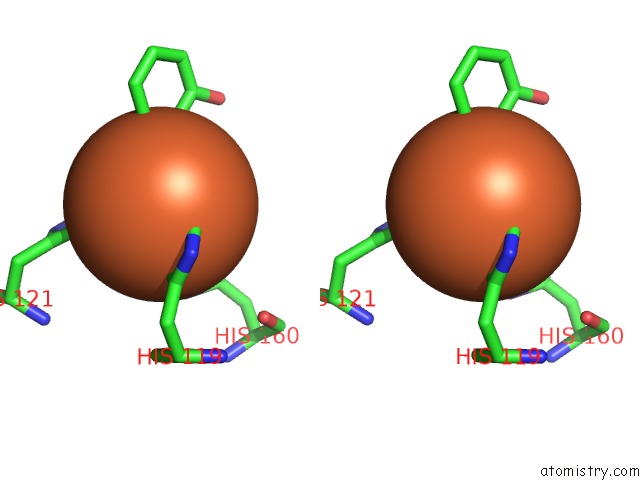
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Salicylate 1,2-Dioxygenase G106A Mutant From Pseudoaminobacter Salicylatoxidans in Complex with Salicylate within 5.0Å range:
|
Reference:
M.Ferraroni,
I.Matera,
S.Burger,
S.Reichert,
L.Steimer,
A.Scozzafava,
A.Stolz,
F.Briganti.
The Salicylate 1,2-Dioxygenase As A Model For A Conventional Gentisate 1,2-Dioxygenase: Crystal Structures of the G106A Mutant and Its Adducts with Gentisate and Salicylate. Febs J. V. 280 1643 2013.
ISSN: ISSN 1742-4658
PubMed: 23384287
DOI: 10.1111/FEBS.12173
Page generated: Tue Aug 5 04:57:42 2025
ISSN: ISSN 1742-4658
PubMed: 23384287
DOI: 10.1111/FEBS.12173
Last articles
Fe in 3VM4Fe in 3VLY
Fe in 3VLX
Fe in 3VLZ
Fe in 3VKT
Fe in 3VLL
Fe in 3VLM
Fe in 3VLK
Fe in 3VLH
Fe in 3VLI