Iron »
PDB 3nxu-3ol5 »
3o1y »
Iron in PDB 3o1y: Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface
Protein crystallography data
The structure of Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface, PDB code: 3o1y
was solved by
M.De March,
R.De Zorzi,
N.Demitri,
C.Gabbiani,
A.Guerri,
A.Casini,
L.Messori,
S.Geremia,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 69.01 / 1.75 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.695, 79.695, 89.247, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.6 / 24.8 |
Iron Binding Sites:
The binding sites of Iron atom in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface
(pdb code 3o1y). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 3 binding sites of Iron where determined in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface, PDB code: 3o1y:
Jump to Iron binding site number: 1; 2; 3;
In total 3 binding sites of Iron where determined in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface, PDB code: 3o1y:
Jump to Iron binding site number: 1; 2; 3;
Iron binding site 1 out of 3 in 3o1y
Go back to
Iron binding site 1 out
of 3 in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface
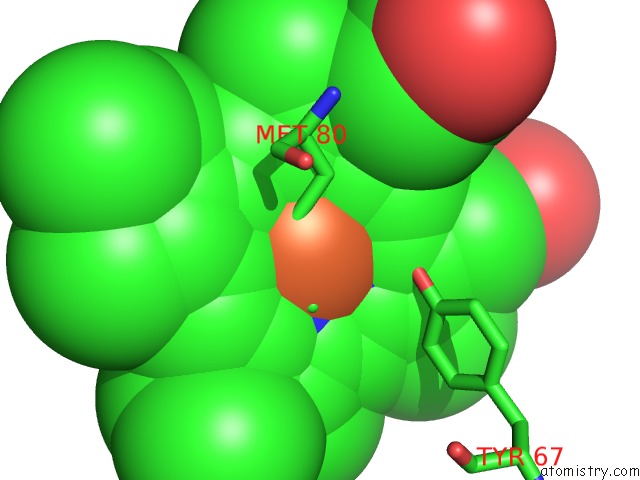
Mono view
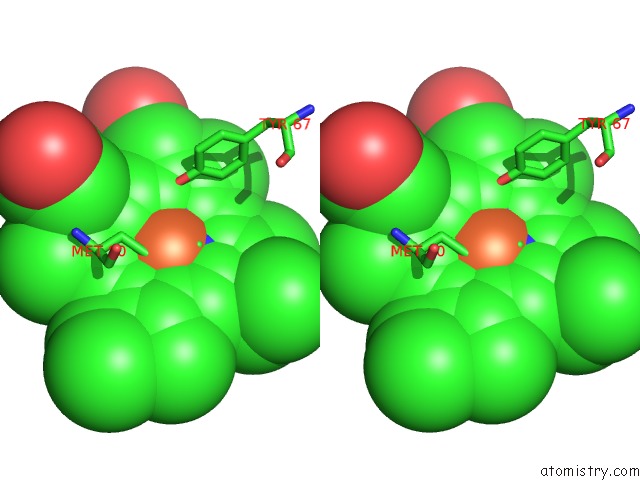
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface within 5.0Å range:
|
Iron binding site 2 out of 3 in 3o1y
Go back to
Iron binding site 2 out
of 3 in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface
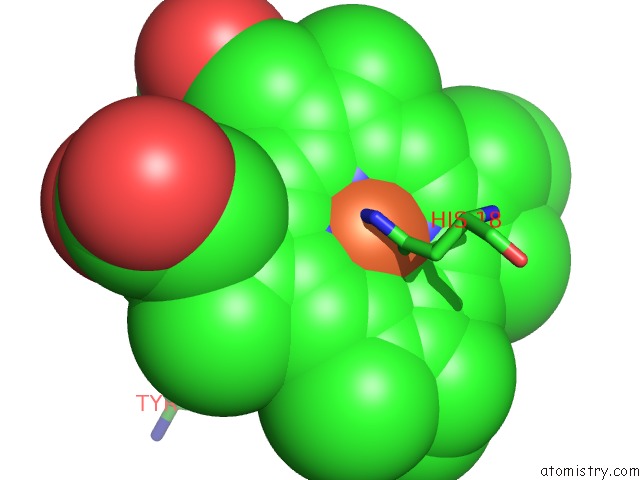
Mono view
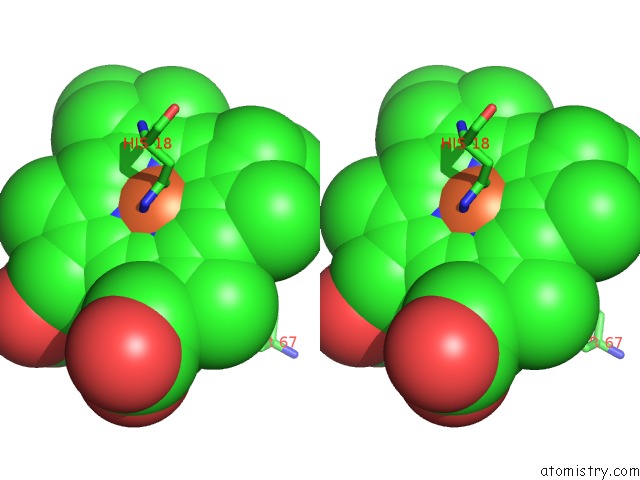
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface within 5.0Å range:
|
Iron binding site 3 out of 3 in 3o1y
Go back to
Iron binding site 3 out
of 3 in the Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface
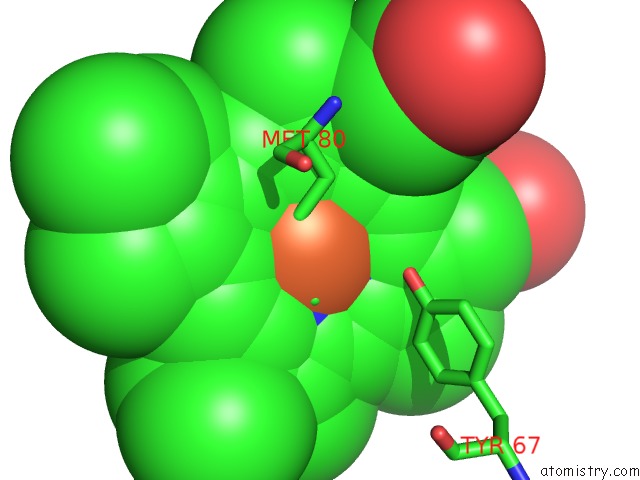
Mono view
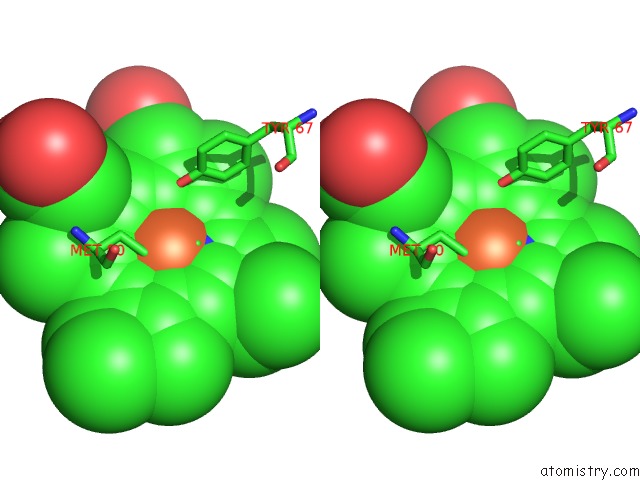
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Electron Transfer Complexes: Experimental Mapping of the Redox- Dependent Cytochrome C Electrostatic Surface within 5.0Å range:
|
Reference:
M.De March,
N.Demitri,
R.De Zorzi,
A.Casini,
C.Gabbiani,
A.Guerri,
L.Messori,
S.Geremia.
Nitrate As A Probe of Cytochrome C Surface: Crystallographic Identification of Crucial "Hot Spots" For Protein-Protein Recognition. J. Inorg. Biochem. V. 135 58 2014.
ISSN: ISSN 1873-3344
PubMed: 24662464
DOI: 10.1016/J.JINORGBIO.2014.02.015
Page generated: Tue Aug 5 05:02:49 2025
ISSN: ISSN 1873-3344
PubMed: 24662464
DOI: 10.1016/J.JINORGBIO.2014.02.015
Last articles
Fe in 3VYTFe in 3VYU
Fe in 3W1W
Fe in 3W08
Fe in 3VYS
Fe in 3VYR
Fe in 3VV9
Fe in 3VVA
Fe in 3VYM
Fe in 3VXJ