Iron »
PDB 3sxv-3tgm »
3tf1 »
Iron in PDB 3tf1: Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon
Protein crystallography data
The structure of Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon, PDB code: 3tf1
was solved by
M.B.Winter,
M.A.Herzik Jr.,
J.Kuriyan,
M.A.Marletta,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.81 / 2.04 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 80.128, 130.214, 42.810, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18 / 22.1 |
Other elements in 3tf1:
The structure of Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon also contains other interesting chemical elements:
| Xenon | (Xe) | 4 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon
(pdb code 3tf1). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon, PDB code: 3tf1:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon, PDB code: 3tf1:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 3tf1
Go back to
Iron binding site 1 out
of 2 in the Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon
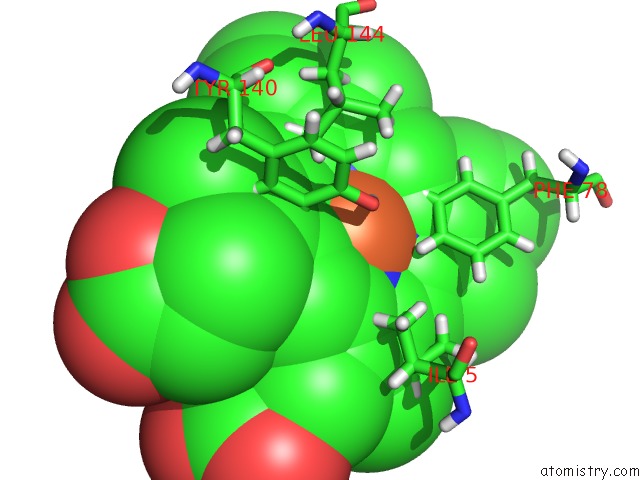
Mono view
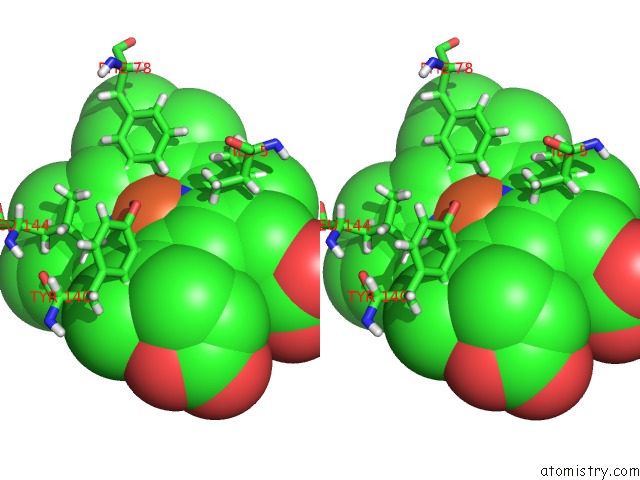
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon within 5.0Å range:
|
Iron binding site 2 out of 2 in 3tf1
Go back to
Iron binding site 2 out
of 2 in the Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon
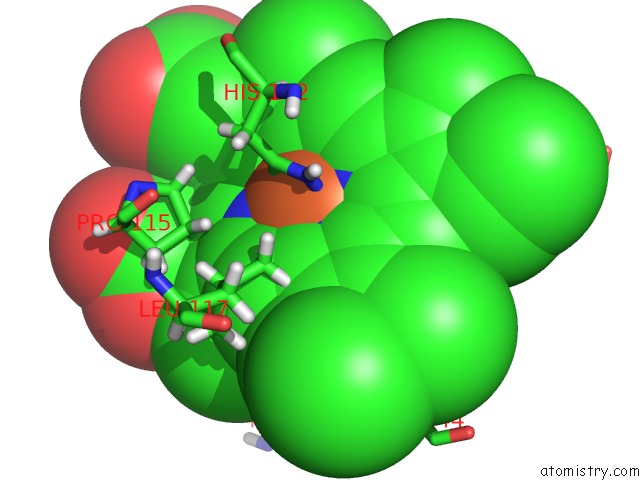
Mono view
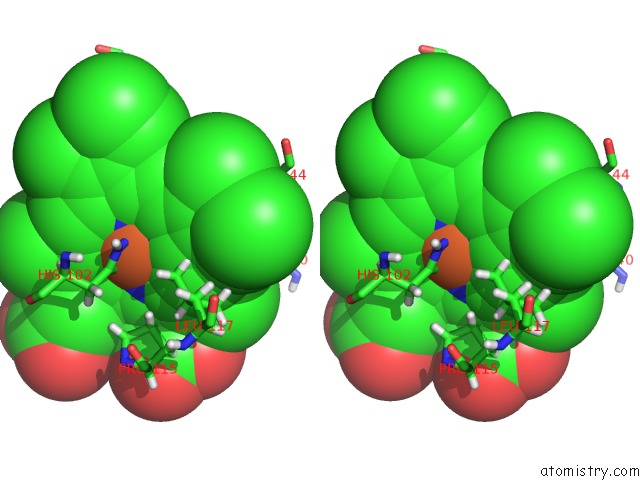
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of An H-Nox Protein From T. Tengcongensis Under 6 Atm of Xenon within 5.0Å range:
|
Reference:
M.B.Winter,
M.A.Herzik,
J.Kuriyan,
M.A.Marletta.
Tunnels Modulate Ligand Flux in A Heme Nitric Oxide/Oxygen Binding (H-Nox) Domain. Proc.Natl.Acad.Sci.Usa V. 108 E881 2011.
ISSN: ISSN 0027-8424
PubMed: 21997213
DOI: 10.1073/PNAS.1114038108
Page generated: Sun Aug 4 20:23:35 2024
ISSN: ISSN 0027-8424
PubMed: 21997213
DOI: 10.1073/PNAS.1114038108
Last articles
F in 4NQAF in 4NMT
F in 4NU1
F in 4NMG
F in 4NLF
F in 4NMS
F in 4NIC
F in 4NLD
F in 4NJU
F in 4NL1