Iron »
PDB 3x20-3zjo »
3zcy »
Iron in PDB 3zcy: Ascorbate Peroxidase W41A-H42Y Mutant
Enzymatic activity of Ascorbate Peroxidase W41A-H42Y Mutant
All present enzymatic activity of Ascorbate Peroxidase W41A-H42Y Mutant:
1.11.1.11;
1.11.1.11;
Protein crystallography data
The structure of Ascorbate Peroxidase W41A-H42Y Mutant, PDB code: 3zcy
was solved by
A.Gumiero,
E.L.Raven,
P.C.E.Moody,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.797 / 2.00 |
| Space group | P 42 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.280, 82.280, 75.170, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.97 / 21.32 |
Other elements in 3zcy:
The structure of Ascorbate Peroxidase W41A-H42Y Mutant also contains other interesting chemical elements:
| Potassium | (K) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Ascorbate Peroxidase W41A-H42Y Mutant
(pdb code 3zcy). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Ascorbate Peroxidase W41A-H42Y Mutant, PDB code: 3zcy:
In total only one binding site of Iron was determined in the Ascorbate Peroxidase W41A-H42Y Mutant, PDB code: 3zcy:
Iron binding site 1 out of 1 in 3zcy
Go back to
Iron binding site 1 out
of 1 in the Ascorbate Peroxidase W41A-H42Y Mutant
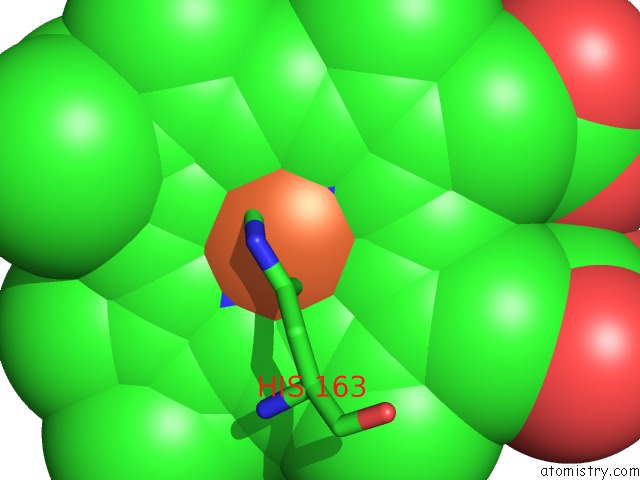
Mono view
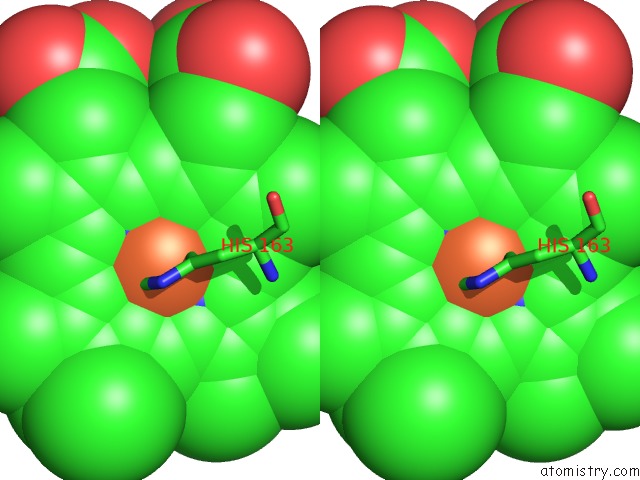
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Ascorbate Peroxidase W41A-H42Y Mutant within 5.0Å range:
|
Reference:
A.Guimero,
S.K.Badyal,
T.Leeks,
P.C.E.Moody,
E.L.Raven.
Probing the Conformational Mobility of the Active Site of Ascorbate Peroxidase Dalton Trans V. 42 3170 2013.
ISSN: ISSN 1477-9226
PubMed: 23202589
DOI: 10.1039/C2DT32455E
Page generated: Tue Aug 5 08:34:11 2025
ISSN: ISSN 1477-9226
PubMed: 23202589
DOI: 10.1039/C2DT32455E
Last articles
Fe in 4UQHFe in 4UPT
Fe in 4UPS
Fe in 4UPR
Fe in 4UPQ
Fe in 4UPP
Fe in 4UPO
Fe in 4UOB
Fe in 4UNF
Fe in 4UPN