Iron »
PDB 4a9v-4b2y »
4ac8 »
Iron in PDB 4ac8: R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
Enzymatic activity of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
All present enzymatic activity of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix:
1.17.4.1;
1.17.4.1;
Protein crystallography data
The structure of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix, PDB code: 4ac8
was solved by
C.S.Andersson,
C.L.Berthold,
M.Hogbom,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.72 / 2.75 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 109.990, 210.850, 139.960, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22 / 26.8 |
Other elements in 4ac8:
The structure of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix also contains other interesting chemical elements:
| Manganese | (Mn) | 4 atoms |
| Calcium | (Ca) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
(pdb code 4ac8). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix, PDB code: 4ac8:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix, PDB code: 4ac8:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 4ac8
Go back to
Iron binding site 1 out
of 4 in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
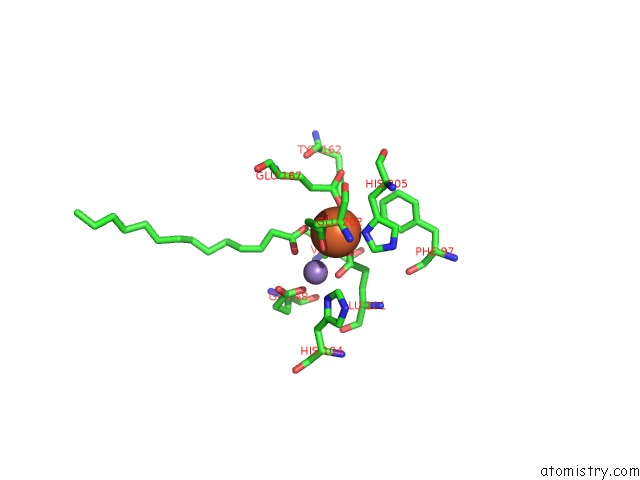
Mono view
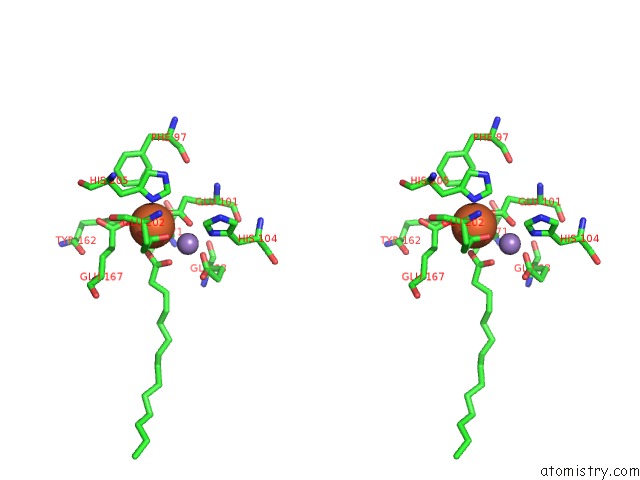
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix within 5.0Å range:
|
Iron binding site 2 out of 4 in 4ac8
Go back to
Iron binding site 2 out
of 4 in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
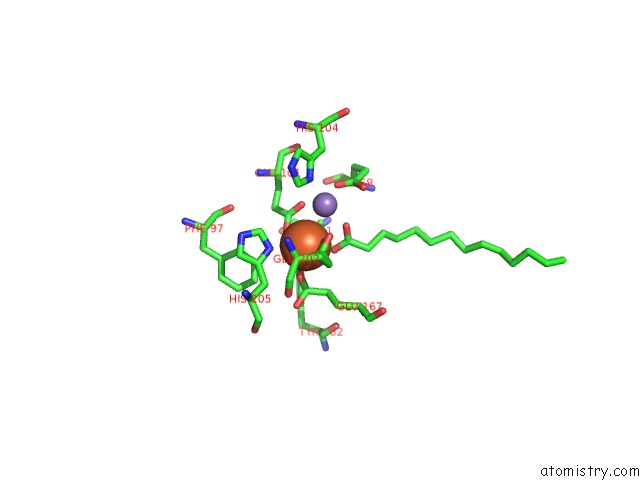
Mono view
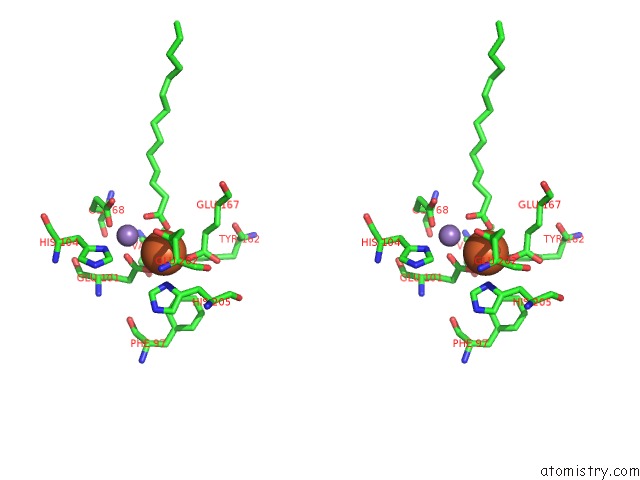
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix within 5.0Å range:
|
Iron binding site 3 out of 4 in 4ac8
Go back to
Iron binding site 3 out
of 4 in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
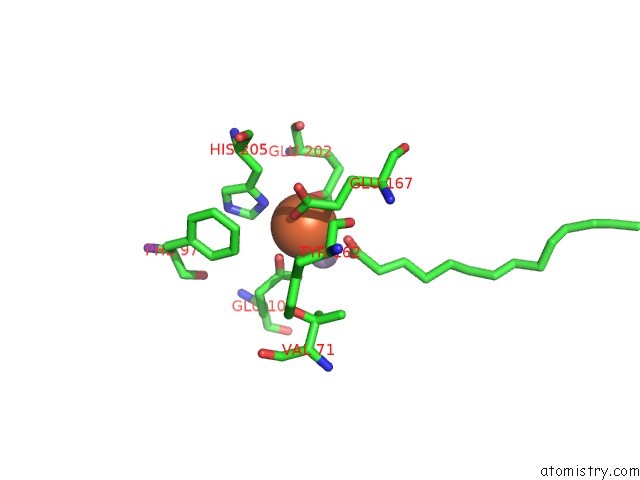
Mono view
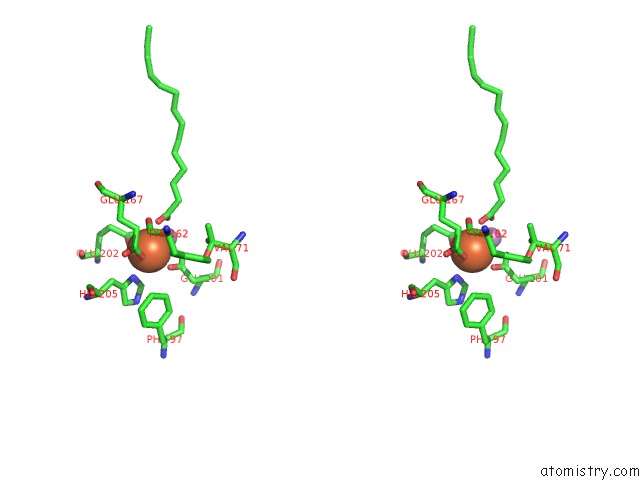
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix within 5.0Å range:
|
Iron binding site 4 out of 4 in 4ac8
Go back to
Iron binding site 4 out
of 4 in the R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix
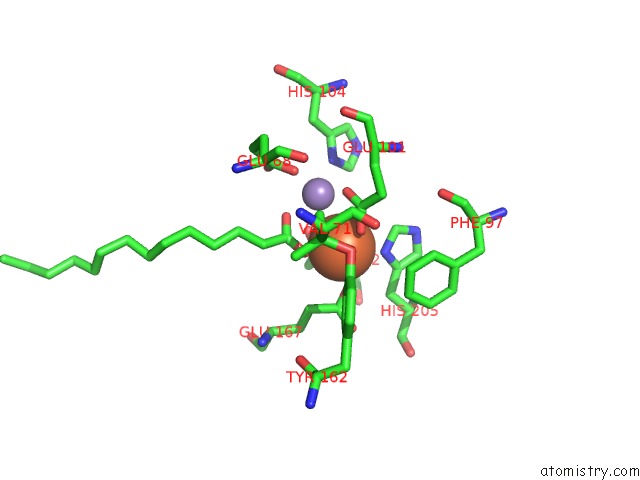
Mono view
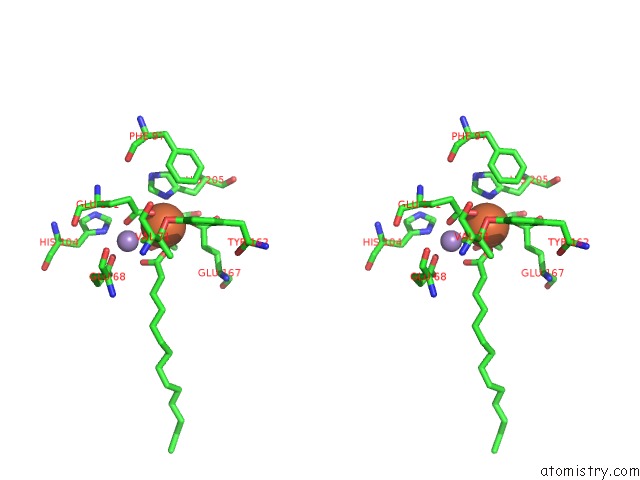
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of R2-Like Ligand Binding Mn-Fe Oxidase From M. Tuberculosis with An Organized C-Terminal Helix within 5.0Å range:
|
Reference:
C.S.Andersson,
C.L.Berthold,
M.Hogbom.
A Dynamic C-Terminal Segment in the Mycobacterium Tuberculosis Mn/Fe R2LOX Protein Can Adopt A Helical Structure with Possible Functional Consequences. Chem.Biodivers. V. 9 1981 2012.
ISSN: ISSN 1612-1872
PubMed: 22976985
DOI: 10.1002/CBDV.201100428
Page generated: Tue Aug 5 08:57:44 2025
ISSN: ISSN 1612-1872
PubMed: 22976985
DOI: 10.1002/CBDV.201100428
Last articles
Fe in 5ADJFe in 5ADF
Fe in 5ADI
Fe in 5ADG
Fe in 5AA5
Fe in 5ADE
Fe in 5ADD
Fe in 5ADC
Fe in 5ADB
Fe in 5ADA