Iron »
PDB 4bmq-4ccp »
4c9k »
Iron in PDB 4c9k: Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1
Protein crystallography data
The structure of Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1, PDB code: 4c9k
was solved by
D.Batabyal,
T.L Poulos,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.530 / 2.18 |
| Space group | P 64 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 151.318, 151.318, 196.141, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 17.53 / 21.81 |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1
(pdb code 4c9k). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1, PDB code: 4c9k:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1, PDB code: 4c9k:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 4c9k
Go back to
Iron binding site 1 out
of 2 in the Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1
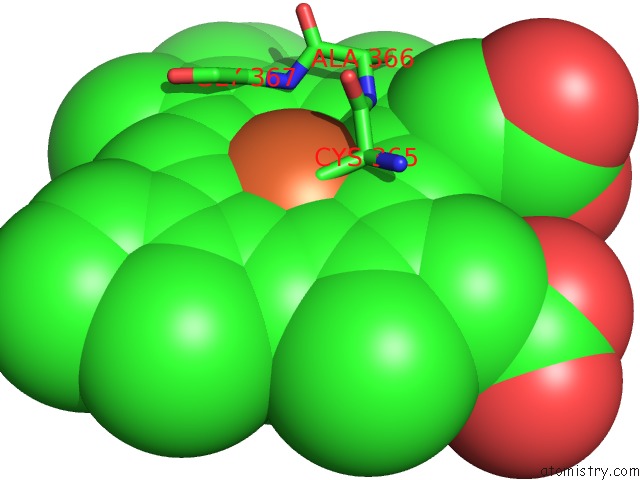
Mono view
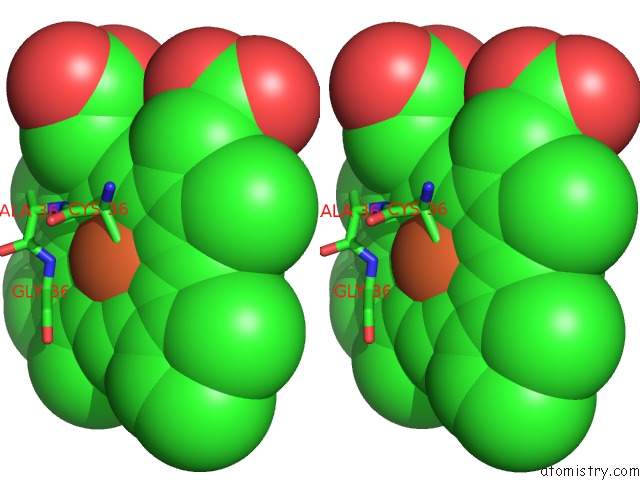
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1 within 5.0Å range:
|
Iron binding site 2 out of 2 in 4c9k
Go back to
Iron binding site 2 out
of 2 in the Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1
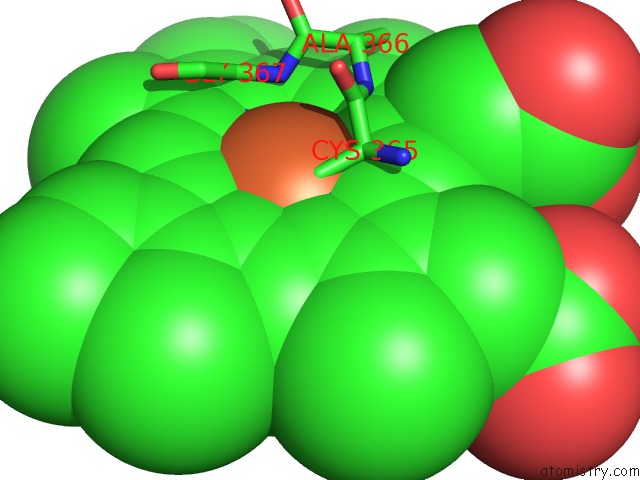
Mono view
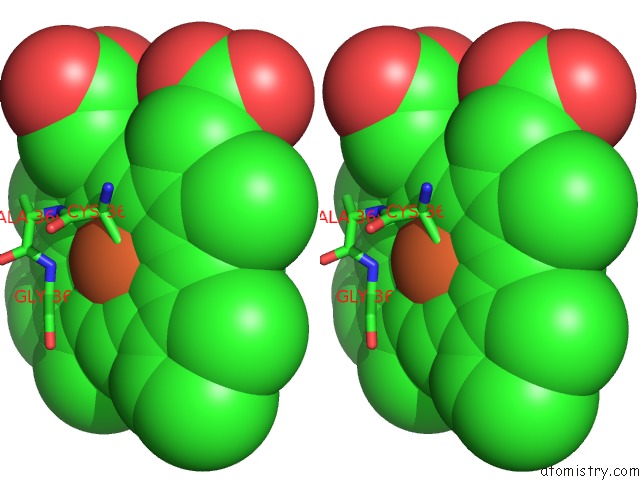
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Camphor and Hydroxycamphor Bound Wild Type CYP101D1 within 5.0Å range:
|
Reference:
D.Batabyal,
T.L.Poulos.
Crystal Structures and Functional Characterization of Wild- Type CYP101D1 and Its Active Site Mutants. Biochemistry V. 52 8898 2013.
ISSN: ISSN 0006-2960
PubMed: 24261604
DOI: 10.1021/BI401330C
Page generated: Tue Aug 5 09:23:52 2025
ISSN: ISSN 0006-2960
PubMed: 24261604
DOI: 10.1021/BI401330C
Last articles
Fe in 5MEDFe in 5MDX
Fe in 5MCS
Fe in 5MAA
Fe in 5MAU
Fe in 5MAB
Fe in 5MBN
Fe in 5MBA
Fe in 5MAP
Fe in 5MA2