Iron »
PDB 4fh7-4g38 »
4fwx »
Iron in PDB 4fwx: Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin)
Protein crystallography data
The structure of Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin), PDB code: 4fwx
was solved by
Y.-G.Gao,
D.Stoner-Ma,
H.Robinson,
I.D.Petrik,
K.D.Miner,
Y.Lu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.57 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 39.219, 47.718, 77.089, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.1 / 26.6 |
Iron Binding Sites:
The binding sites of Iron atom in the Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin)
(pdb code 4fwx). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin), PDB code: 4fwx:
In total only one binding site of Iron was determined in the Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin), PDB code: 4fwx:
Iron binding site 1 out of 1 in 4fwx
Go back to
Iron binding site 1 out
of 1 in the Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin)
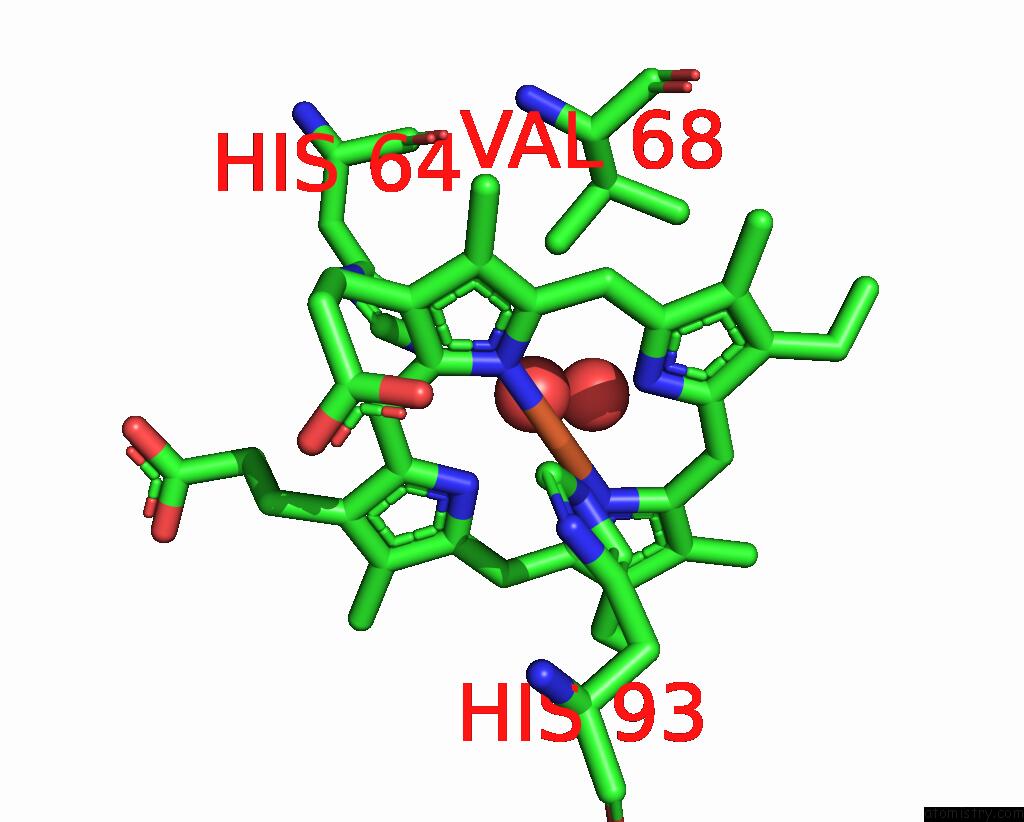
Mono view
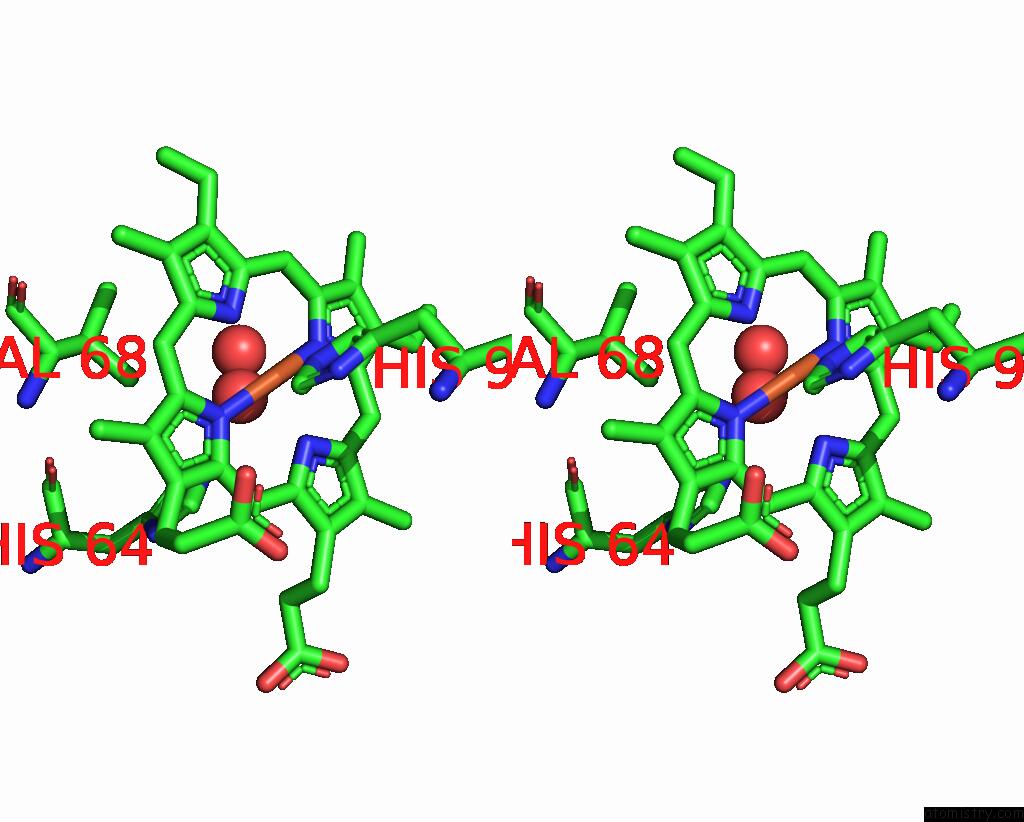
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Aquoferric F33Y Cub Myoglobin (F33Y L29H F43H Sperm Whale Myoglobin) within 5.0Å range:
|
Reference:
K.D.Miner,
A.Mukherjee,
Y.G.Gao,
E.L.Null,
I.D.Petrik,
X.Zhao,
N.Yeung,
H.Robinson,
Y.Lu.
A Designed Functional Metalloenzyme That Reduces O(2) to H(2) O with Over One Thousand Turnovers. Angew.Chem.Int.Ed.Engl. V. 51 5589 2012.
ISSN: ISSN 1433-7851
PubMed: 22539151
DOI: 10.1002/ANIE.201201981
Page generated: Tue Aug 5 10:30:36 2025
ISSN: ISSN 1433-7851
PubMed: 22539151
DOI: 10.1002/ANIE.201201981
Last articles
Fe in 5TH5Fe in 5T5M
Fe in 5TIA
Fe in 5TI9
Fe in 5TGS
Fe in 5TFU
Fe in 5TG0
Fe in 5TFT
Fe in 5TE8
Fe in 5TDV