Iron »
PDB 4g39-4gl5 »
4g51 »
Iron in PDB 4g51: Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
Protein crystallography data
The structure of Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State)., PDB code: 4g51
was solved by
A.Merlino,
A.Balsamo,
A.Pica,
L.Mazzarella,
A.Vergara,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 2.50 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.002, 94.672, 61.956, 90.00, 89.16, 90.00 |
| R / Rfree (%) | 18.1 / 24.9 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
(pdb code 4g51). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State)., PDB code: 4g51:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State)., PDB code: 4g51:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 4g51
Go back to
Iron binding site 1 out
of 4 in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
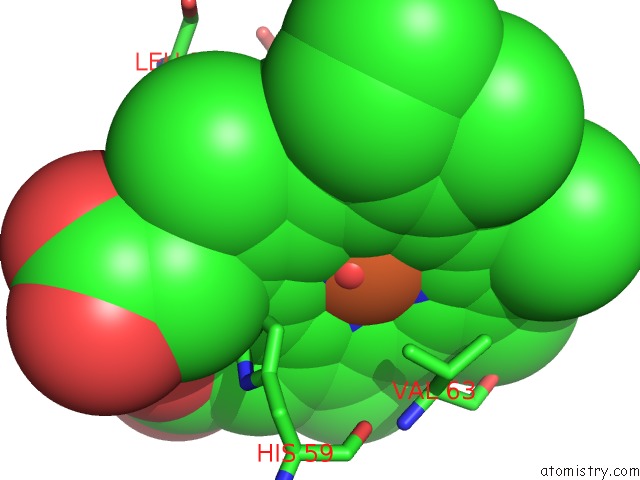
Mono view
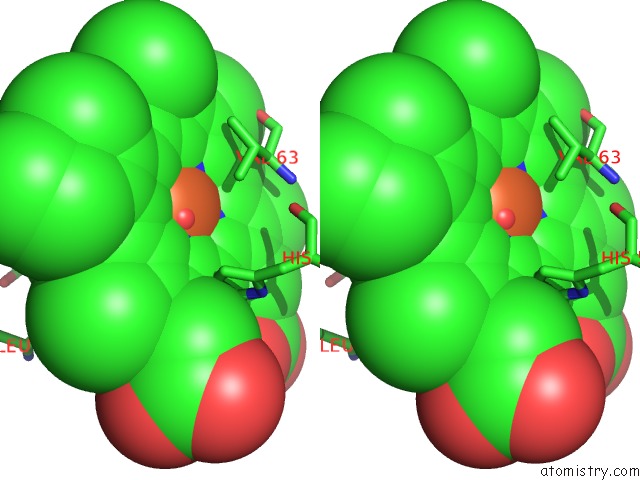
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State). within 5.0Å range:
|
Iron binding site 2 out of 4 in 4g51
Go back to
Iron binding site 2 out
of 4 in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
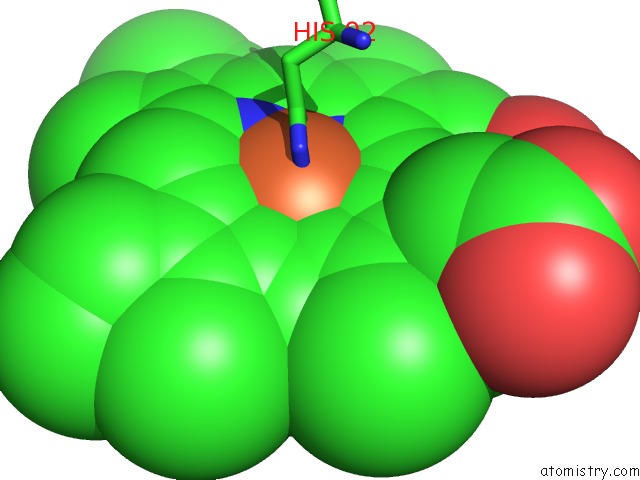
Mono view
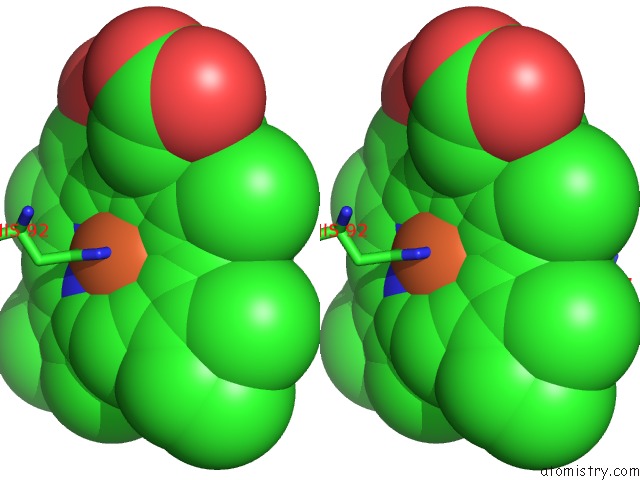
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State). within 5.0Å range:
|
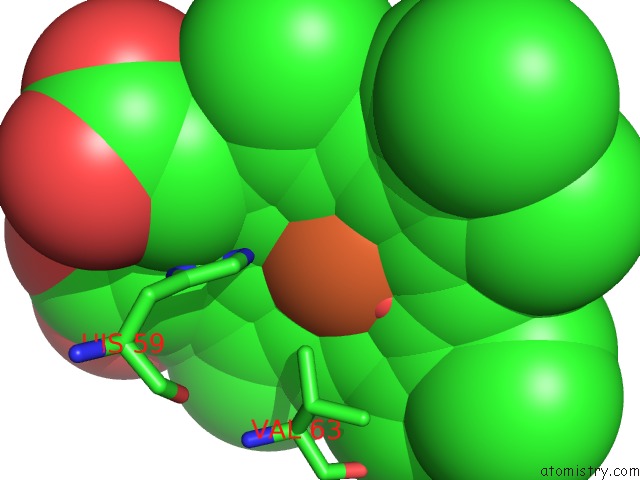
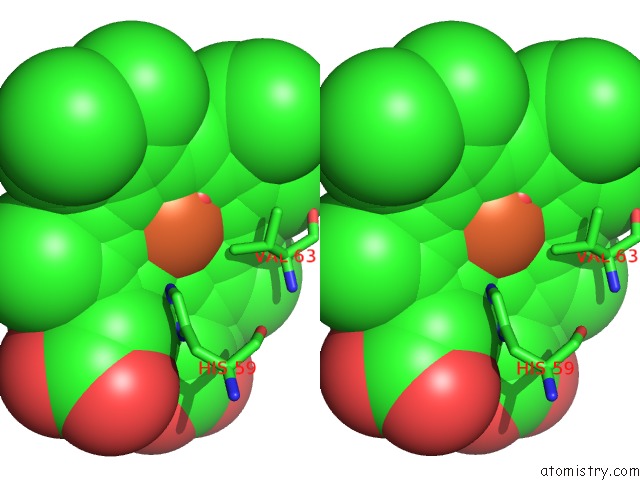
Iron binding site 3 out of 4 in 4g51
Go back to
Iron binding site 3 out
of 4 in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State). within 5.0Å range:
|
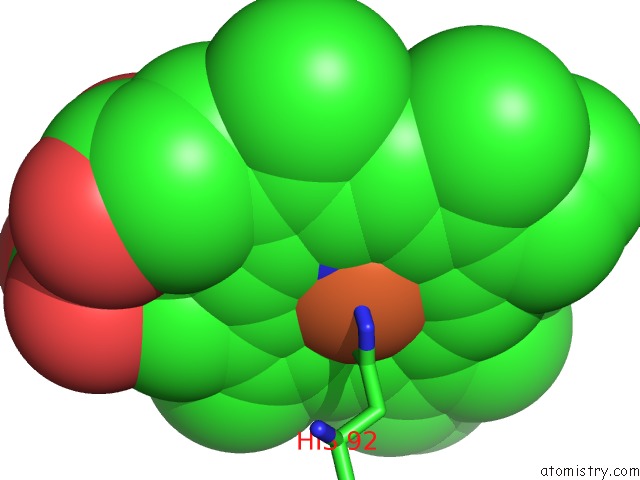
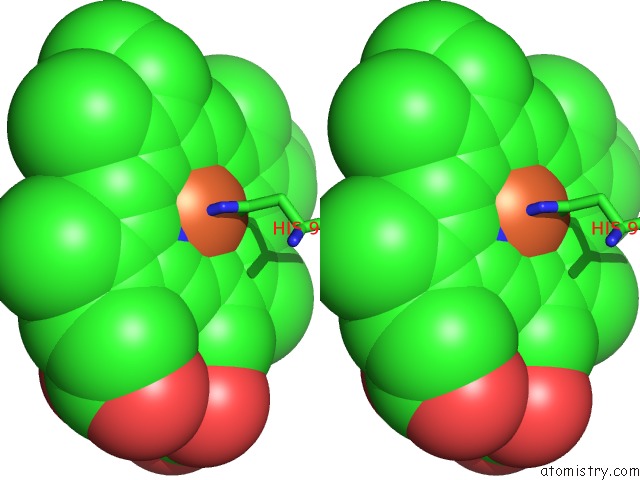
Iron binding site 4 out of 4 in 4g51
Go back to
Iron binding site 4 out
of 4 in the Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State).
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystallographic Analysis of the Interaction of Nitric Oxide with Hemoglobin From Trematomus Bernacchii in the T Quaternary Structure (Fully Ligated State). within 5.0Å range:
|
Reference:
A.Merlino,
M.R.Fuchs,
A.Pica,
A.Balsamo,
F.S.Dworkowski,
G.Pompidor,
L.Mazzarella,
A.Vergara.
Selective X-Ray-Induced No Photodissociation in Haemoglobin Crystals: Evidence From A Raman-Assisted Crystallographic Study. Acta Crystallogr.,Sect.D V. 69 137 2013.
ISSN: ISSN 0907-4449
PubMed: 23275172
DOI: 10.1107/S0907444912042229
Page generated: Mon Aug 5 02:36:12 2024
ISSN: ISSN 0907-4449
PubMed: 23275172
DOI: 10.1107/S0907444912042229
Last articles
F in 7R3VF in 7R7K
F in 7QZ9
F in 7QZ8
F in 7R2B
F in 7R26
F in 7QZ6
F in 7QZ2
F in 7QXL
F in 7QZ5