Iron »
PDB 4k5e-4kf2 »
4k5j »
Iron in PDB 4k5j: Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine
Enzymatic activity of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine
All present enzymatic activity of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine:
1.14.13.39;
1.14.13.39;
Protein crystallography data
The structure of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine, PDB code: 4k5j
was solved by
H.Li,
T.L.Poulos,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.93 / 2.36 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 57.813, 106.289, 156.720, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.1 / 21.4 |
Other elements in 4k5j:
The structure of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine also contains other interesting chemical elements:
| Arsenic | (As) | 2 atoms |
| Zinc | (Zn) | 1 atom |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine
(pdb code 4k5j). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine, PDB code: 4k5j:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine, PDB code: 4k5j:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 4k5j
Go back to
Iron binding site 1 out
of 2 in the Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine
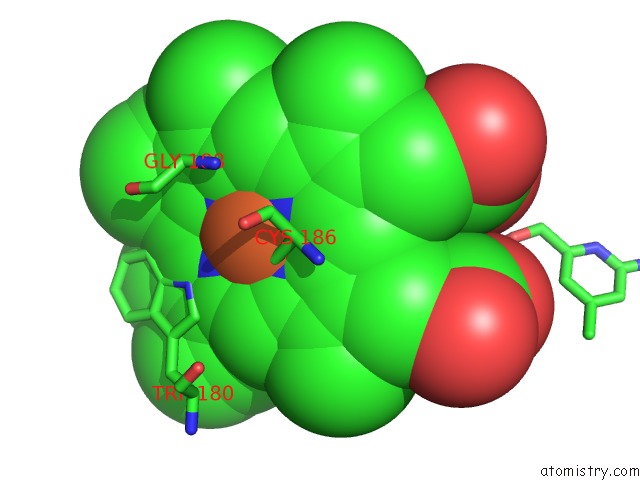
Mono view
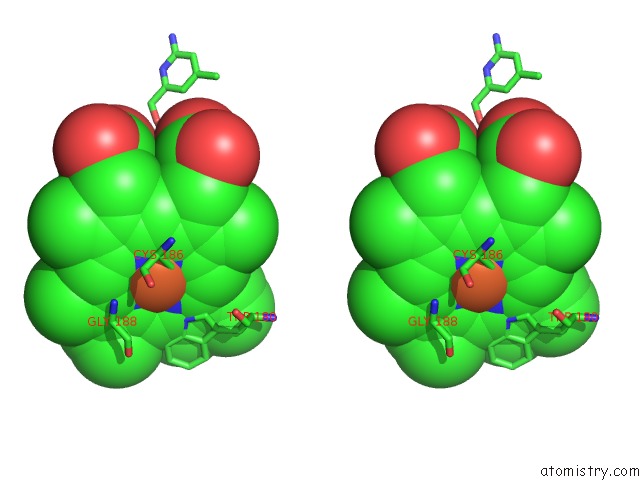
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine within 5.0Å range:
|
Iron binding site 2 out of 2 in 4k5j
Go back to
Iron binding site 2 out
of 2 in the Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine
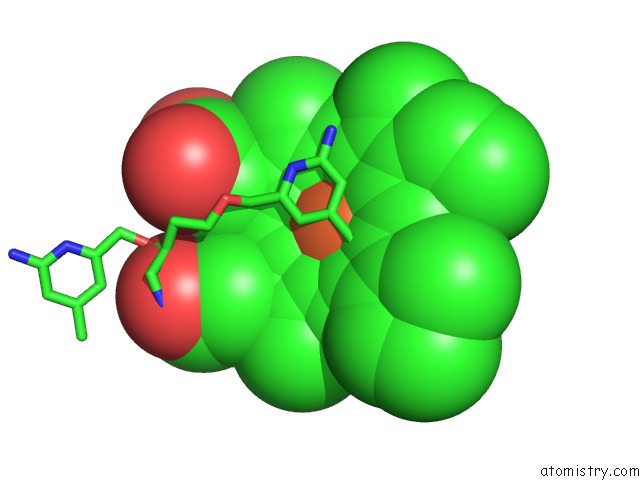
Mono view
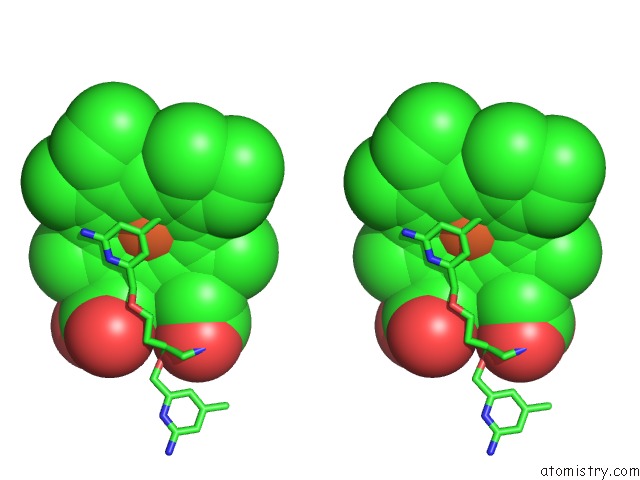
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of Bovine Endothelial Nitric Oxide Synthase Heme Domain in Complex with (S)-1,3-Bis((2-Amino-4-Methylpyridin-6-Yl)-Methoxy)- Butan-4-Amine within 5.0Å range:
|
Reference:
Q.Jing,
H.Li,
G.Chreifi,
L.J.Roman,
P.Martasek,
T.L.Poulos,
R.B.Silverman.
Chiral Linkers to Improve Selectivity of Double-Headed Neuronal Nitric Oxide Synthase Inhibitors. Bioorg.Med.Chem.Lett. V. 23 5674 2013.
ISSN: ISSN 0960-894X
PubMed: 23993333
DOI: 10.1016/J.BMCL.2013.08.034
Page generated: Mon Aug 5 05:17:32 2024
ISSN: ISSN 0960-894X
PubMed: 23993333
DOI: 10.1016/J.BMCL.2013.08.034
Last articles
F in 7NWKF in 7NWA
F in 7NVN
F in 7NVM
F in 7NW2
F in 7NW0
F in 7NVL
F in 7NVX
F in 7NVV
F in 7NVO