Iron »
PDB 4l0d-4lji »
4l2d »
Iron in PDB 4l2d: X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
Enzymatic activity of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
All present enzymatic activity of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis:
1.15.1.1;
1.15.1.1;
Protein crystallography data
The structure of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis, PDB code: 4l2d
was solved by
I.Russo Krauss,
A.Merlino,
F.Sica,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.20 / 2.07 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.303, 103.625, 89.339, 90.00, 103.68, 90.00 |
| R / Rfree (%) | 20 / 22 |
Iron Binding Sites:
The binding sites of Iron atom in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
(pdb code 4l2d). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis, PDB code: 4l2d:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis, PDB code: 4l2d:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 4l2d
Go back to
Iron binding site 1 out
of 4 in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
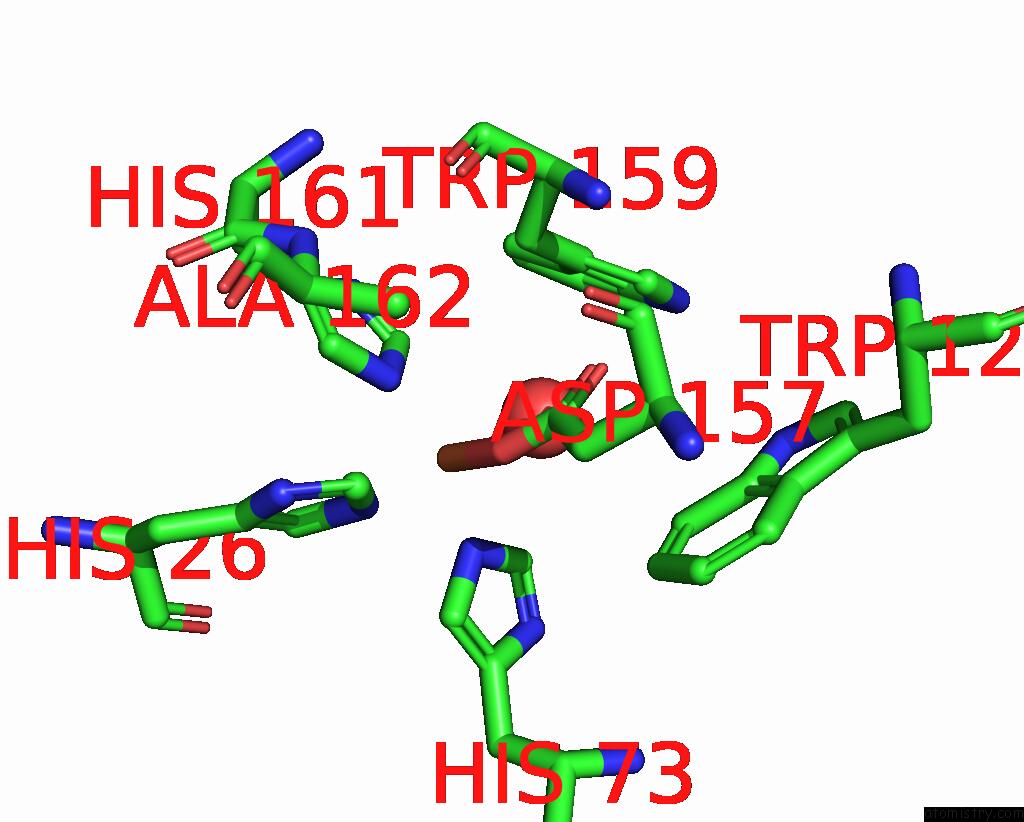
Mono view
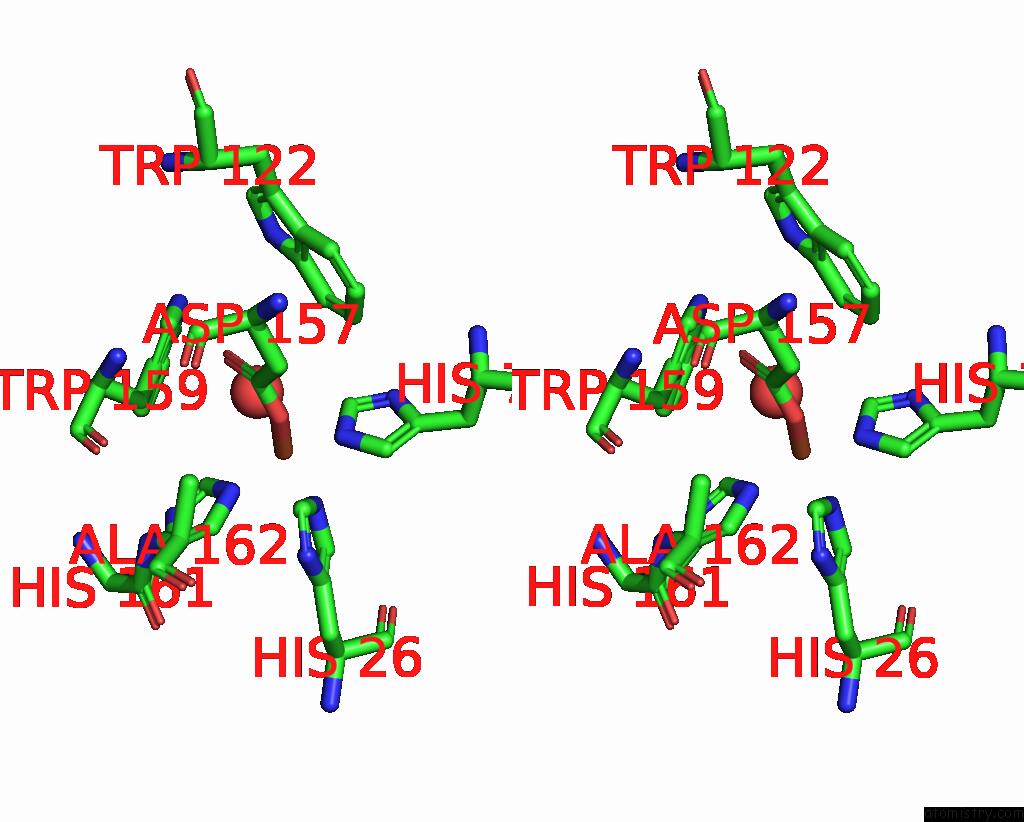
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis within 5.0Å range:
|
Iron binding site 2 out of 4 in 4l2d
Go back to
Iron binding site 2 out
of 4 in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
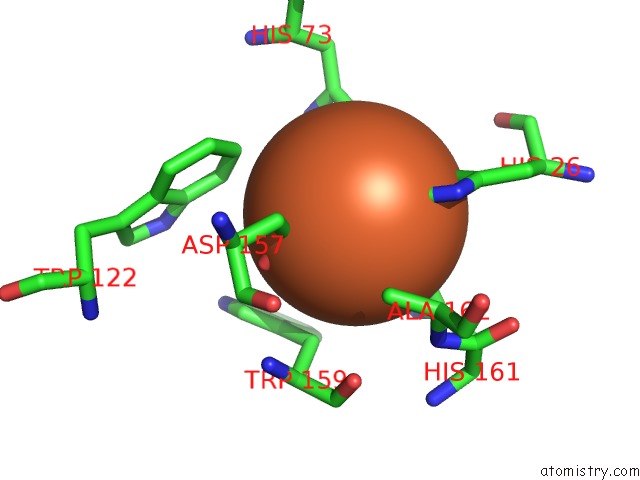
Mono view
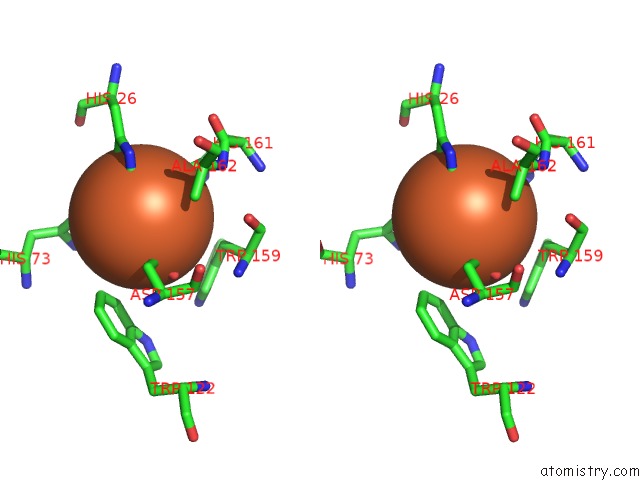
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis within 5.0Å range:
|
Iron binding site 3 out of 4 in 4l2d
Go back to
Iron binding site 3 out
of 4 in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
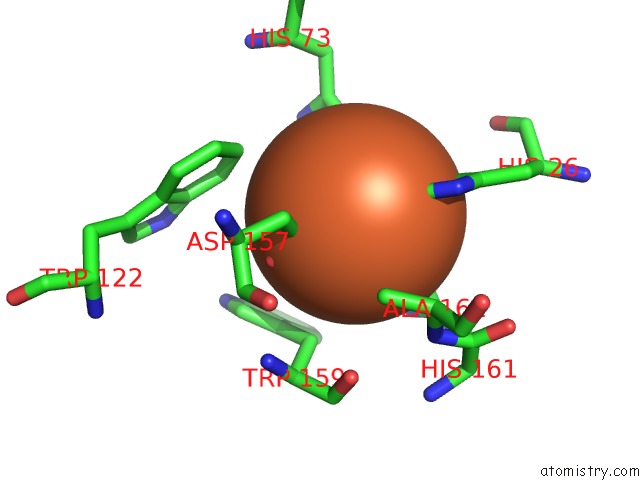
Mono view
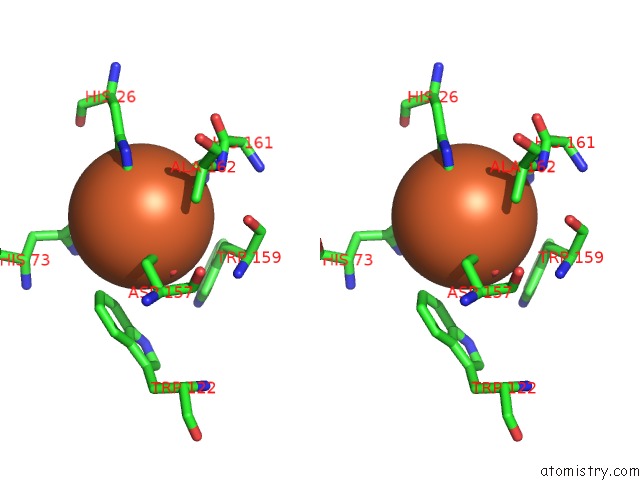
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis within 5.0Å range:
|
Iron binding site 4 out of 4 in 4l2d
Go back to
Iron binding site 4 out
of 4 in the X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis
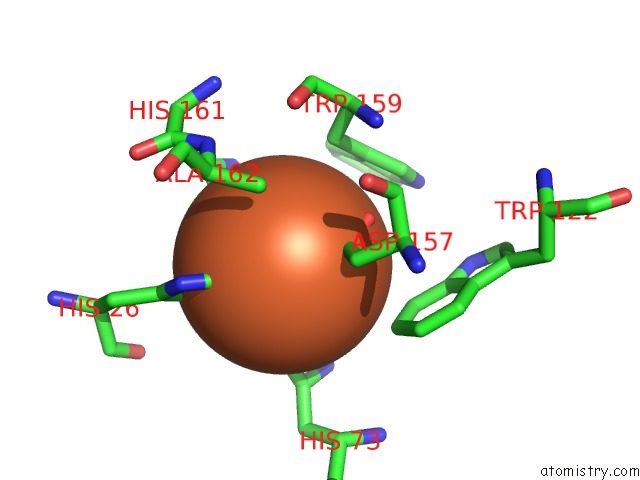
Mono view
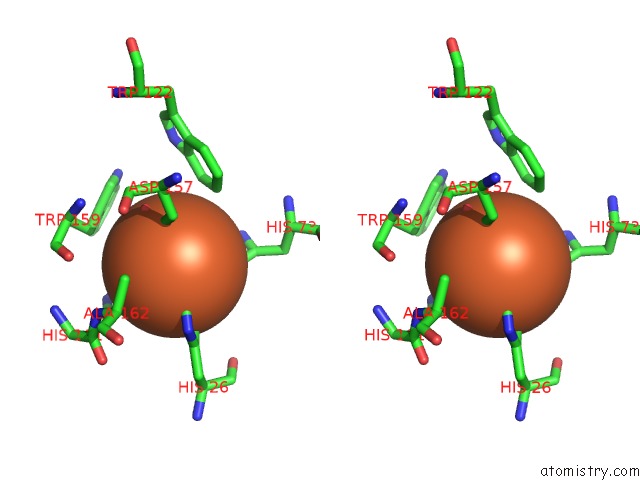
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of X-Ray Structure of the Fe(II) Form of the Iron Superoxide Dismutase From Pseudoalteromonas Haloplanktis within 5.0Å range:
|
Reference:
A.Merlino,
I.Russo Krauss,
I.Castellano,
M.R.Ruocco,
A.Capasso,
E.De Vendittis,
B.Rossi,
F.Sica.
Structural and Denaturation Studies of Two Mutants of A Cold Adapted Superoxide Dismutase Point to the Importance of Electrostatic Interactions in Protein Stability. Biochim.Biophys.Acta V.1844 632 2014.
ISSN: ISSN 0006-3002
PubMed: 24440460
DOI: 10.1016/J.BBAPAP.2014.01.007
Page generated: Tue Aug 5 12:21:55 2025
ISSN: ISSN 0006-3002
PubMed: 24440460
DOI: 10.1016/J.BBAPAP.2014.01.007
Last articles
Fe in 4S3BFe in 4S3A
Fe in 4S39
Fe in 4S38
Fe in 4S2A
Fe in 4S26
Fe in 4S36
Fe in 4S28
Fe in 4S23
Fe in 4S29