Iron »
PDB 5dab-5eax »
5e83 »
Iron in PDB 5e83: Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
Protein crystallography data
The structure of Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment, PDB code: 5e83
was solved by
L.Patskovska,
Y.Patskovsky,
J.B.Bonanno,
S.C.Almo,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.71 / 1.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.777, 59.104, 170.260, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.7 / 20.8 |
Iron Binding Sites:
The binding sites of Iron atom in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
(pdb code 5e83). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment, PDB code: 5e83:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment, PDB code: 5e83:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 5e83
Go back to
Iron binding site 1 out
of 4 in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
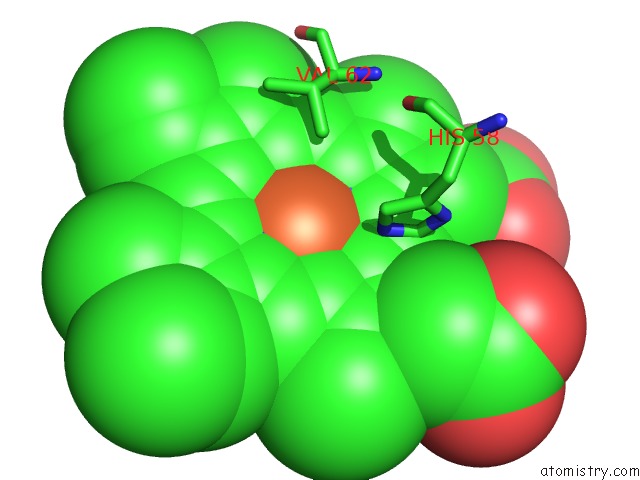
Mono view
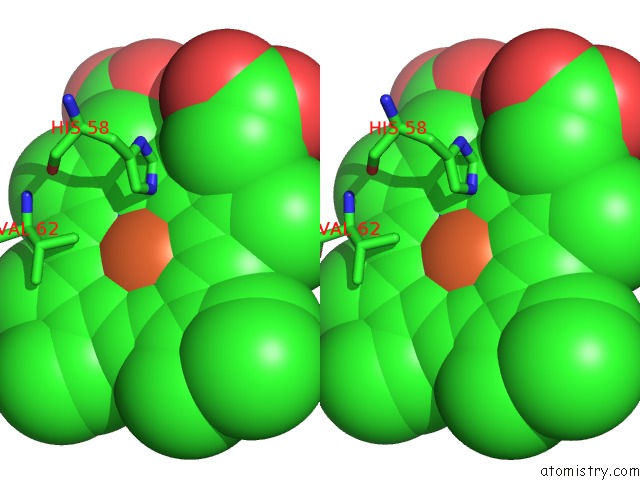
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment within 5.0Å range:
|
Iron binding site 2 out of 4 in 5e83
Go back to
Iron binding site 2 out
of 4 in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
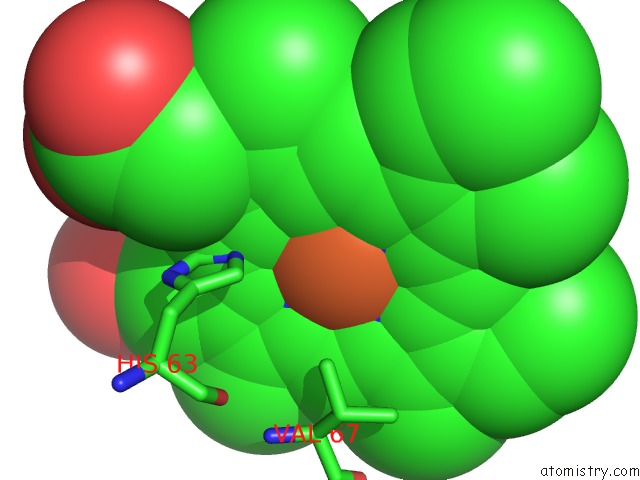
Mono view
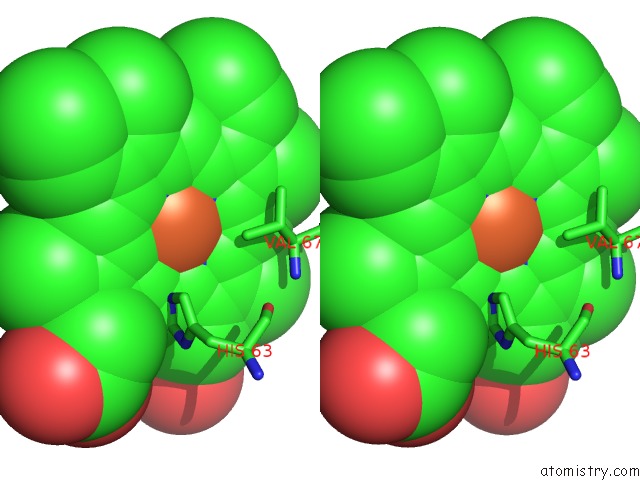
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment within 5.0Å range:
|
Iron binding site 3 out of 4 in 5e83
Go back to
Iron binding site 3 out
of 4 in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
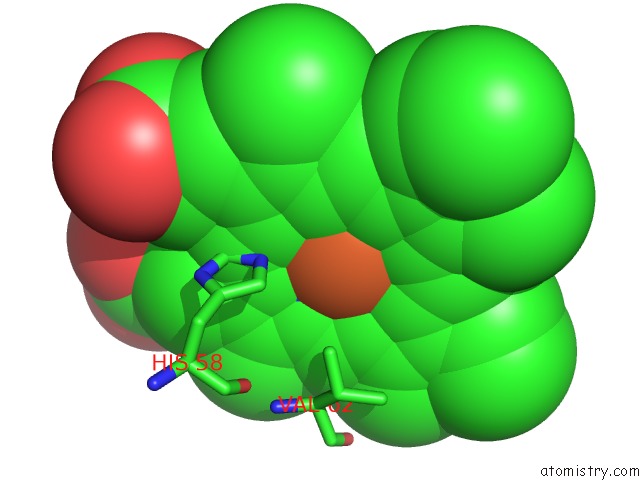
Mono view
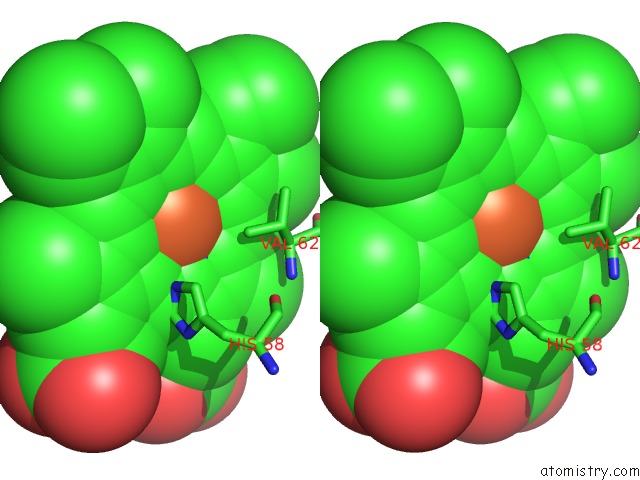
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment within 5.0Å range:
|
Iron binding site 4 out of 4 in 5e83
Go back to
Iron binding site 4 out
of 4 in the Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment
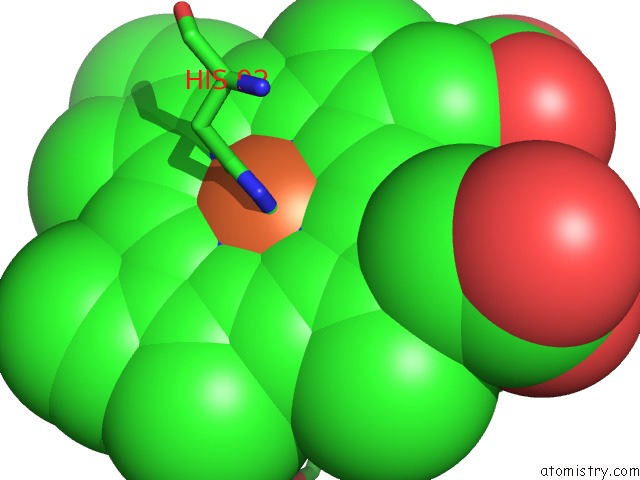
Mono view
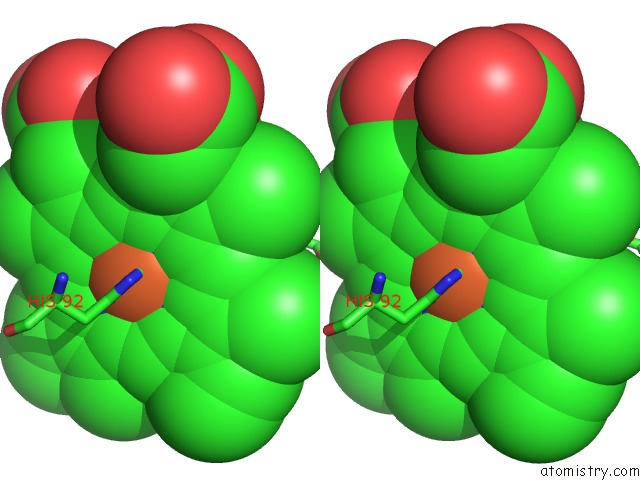
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Crystal Structure of Carbonmonoxy Hemoglobin S (Liganded Sickle Cell Hemoglobin) Complexed with GBT440, Co-Crystallization Experiment within 5.0Å range:
|
Reference:
D.Oksenberg,
K.Dufu,
M.P.Patel,
C.Chuang,
Z.Li,
Q.Xu,
A.Silva-Garcia,
C.Zhou,
A.Hutchaleelaha,
L.Patskovska,
Y.Patskovsky,
S.C.Almo,
U.Sinha,
B.W.Metcalf,
D.R.Archer.
GBT440 Increases Haemoglobin Oxygen Affinity, Reduces Sickling and Prolongs Rbc Half-Life in A Murine Model of Sickle Cell Disease. Br.J.Haematol. V. 175 141 2016.
ISSN: ISSN 0007-1048
PubMed: 27378309
DOI: 10.1111/BJH.14214
Page generated: Tue Aug 5 20:55:07 2025
ISSN: ISSN 0007-1048
PubMed: 27378309
DOI: 10.1111/BJH.14214
Last articles
Fe in 5MDLFe in 5MDJ
Fe in 5MDK
Fe in 5MFA
Fe in 5MEF
Fe in 5MEE
Fe in 5MED
Fe in 5MDX
Fe in 5MCS
Fe in 5MAA