Iron »
PDB 5edt-5ex9 »
5esn »
Iron in PDB 5esn: Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure
Enzymatic activity of Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure
All present enzymatic activity of Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure:
1.14.13.70;
1.14.13.70;
Protein crystallography data
The structure of Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure, PDB code: 5esn
was solved by
A.Sagatova,
M.V.Keniya,
R.K.Wilson,
M.Sabherwal,
J.D.A.Tyndall,
B.C.Monk,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.30 / 2.35 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.520, 65.950, 80.950, 90.00, 98.84, 90.00 |
| R / Rfree (%) | 18.4 / 22.8 |
Iron Binding Sites:
The binding sites of Iron atom in the Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure
(pdb code 5esn). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total only one binding site of Iron was determined in the Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure, PDB code: 5esn:
In total only one binding site of Iron was determined in the Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure, PDB code: 5esn:
Iron binding site 1 out of 1 in 5esn
Go back to
Iron binding site 1 out
of 1 in the Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure
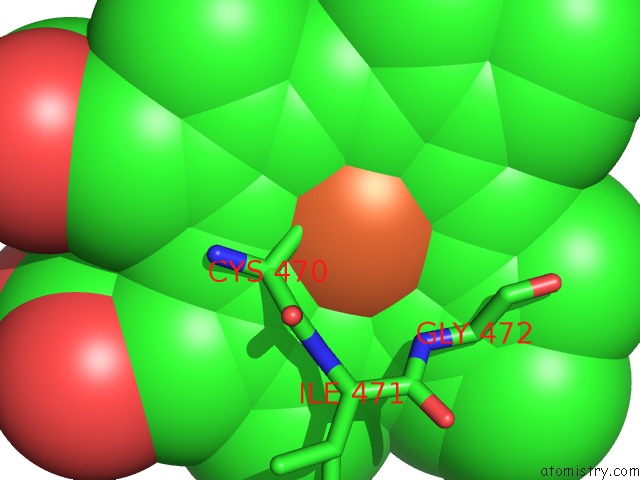
Mono view
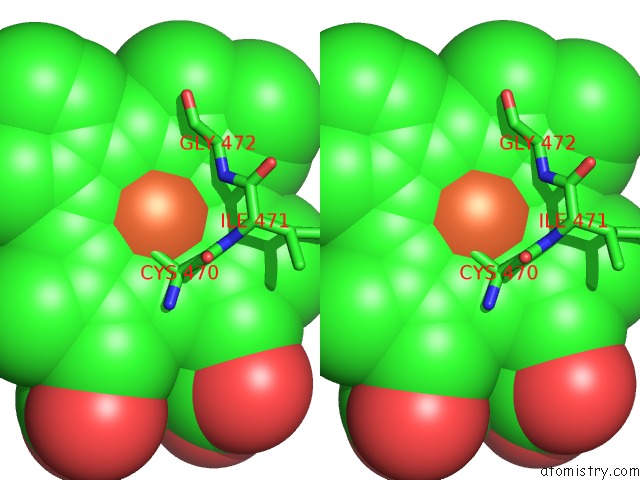
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Saccharomyces Cerevisiae CYP51 (Lanosterol 14-Alpha Demethylase) T322I Mutant Structure within 5.0Å range:
|
Reference:
A.Sagatova,
M.V.Keniya,
R.K.Wilson,
M.Sabherwal,
J.D.A.Tyndall,
B.C.Monk.
Structures of Lanosterol 14-Alpha Demethylase Mutants. To Be Published.
Page generated: Tue Aug 5 21:12:06 2025
Last articles
Fe in 5MEDFe in 5MDX
Fe in 5MCS
Fe in 5MAA
Fe in 5MAU
Fe in 5MAB
Fe in 5MBN
Fe in 5MBA
Fe in 5MAP
Fe in 5MA2