Iron »
PDB 5o17-5ok4 »
5of4 »
Iron in PDB 5of4: The Cryo-Em Structure of Human Tfiih
Enzymatic activity of The Cryo-Em Structure of Human Tfiih
All present enzymatic activity of The Cryo-Em Structure of Human Tfiih:
3.6.4.12;
3.6.4.12;
Iron Binding Sites:
The binding sites of Iron atom in the The Cryo-Em Structure of Human Tfiih
(pdb code 5of4). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the The Cryo-Em Structure of Human Tfiih, PDB code: 5of4:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the The Cryo-Em Structure of Human Tfiih, PDB code: 5of4:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 5of4
Go back to
Iron binding site 1 out
of 4 in the The Cryo-Em Structure of Human Tfiih
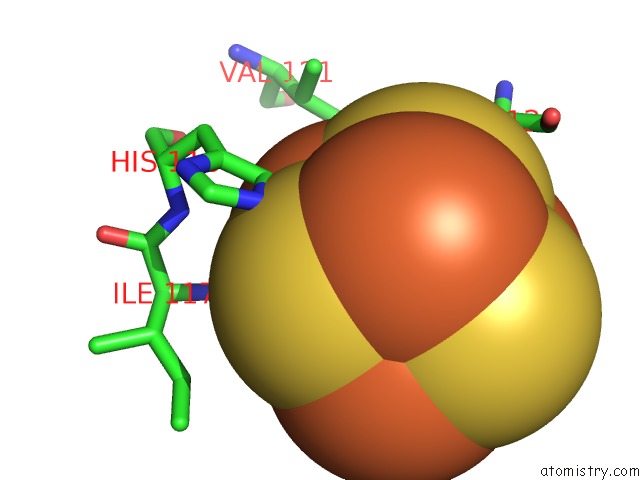
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of The Cryo-Em Structure of Human Tfiih within 5.0Å range:
|
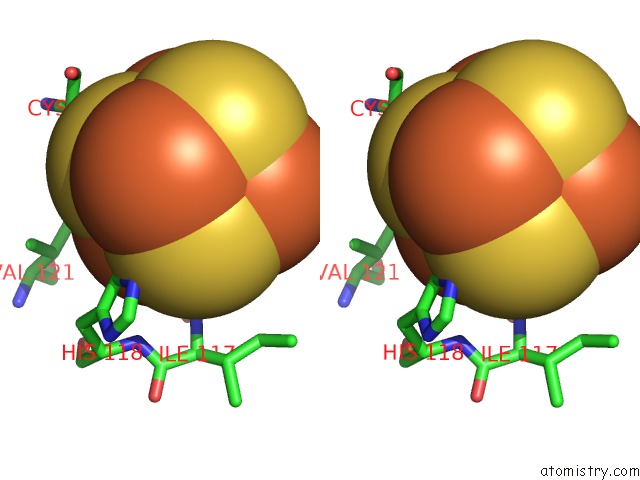
Iron binding site 2 out of 4 in 5of4
Go back to
Iron binding site 2 out
of 4 in the The Cryo-Em Structure of Human Tfiih
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of The Cryo-Em Structure of Human Tfiih within 5.0Å range:
|
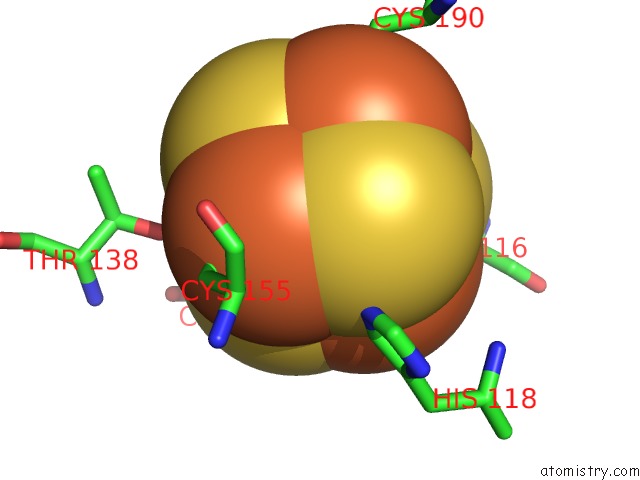
Iron binding site 3 out of 4 in 5of4
Go back to
Iron binding site 3 out
of 4 in the The Cryo-Em Structure of Human Tfiih
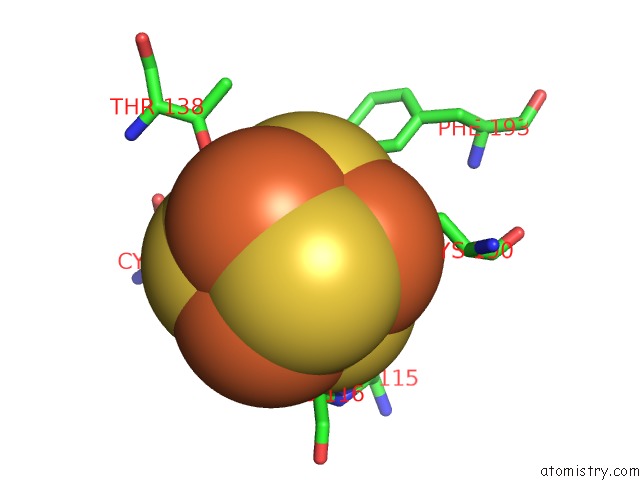
Mono view
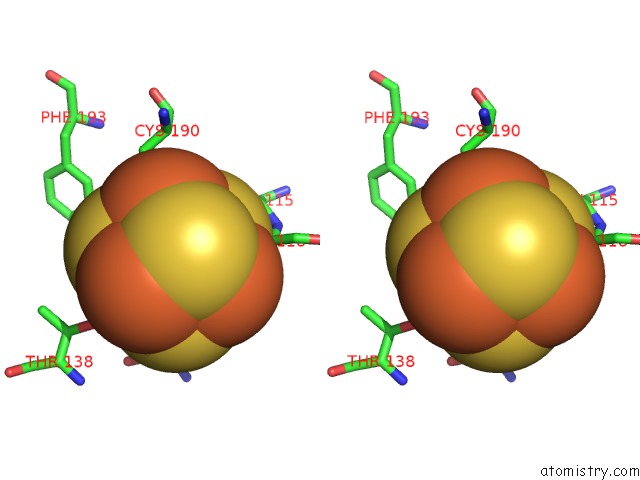
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of The Cryo-Em Structure of Human Tfiih within 5.0Å range:
|
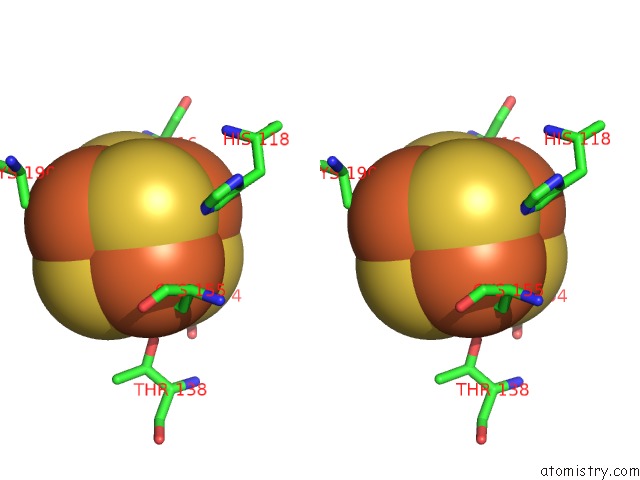
Iron binding site 4 out of 4 in 5of4
Go back to
Iron binding site 4 out
of 4 in the The Cryo-Em Structure of Human Tfiih
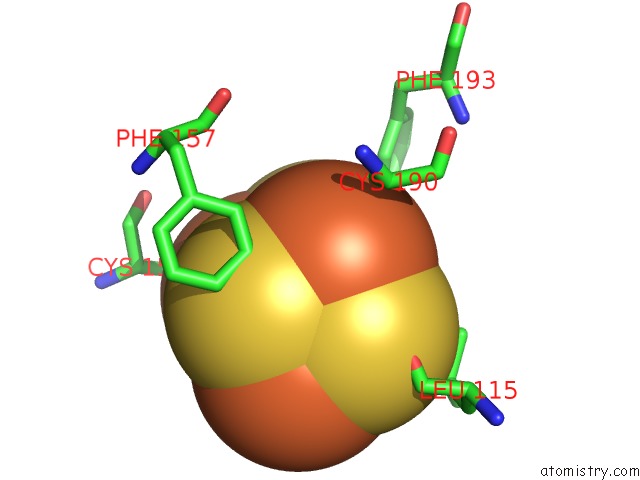
Mono view
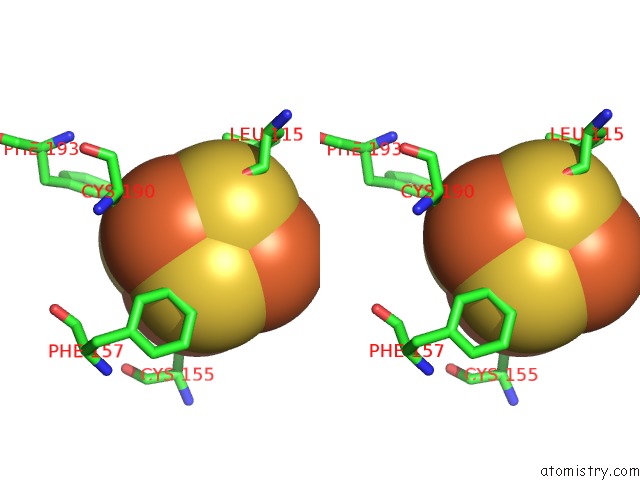
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of The Cryo-Em Structure of Human Tfiih within 5.0Å range:
|
Reference:
B.J.Greber,
T.H.D.Nguyen,
J.Fang,
P.V.Afonine,
P.D.Adams,
E.Nogales.
The Cryo-Electron Microscopy Structure of Human Transcription Factor Iih. Nature V. 549 414 2017.
ISSN: ESSN 1476-4687
PubMed: 28902838
DOI: 10.1038/NATURE23903
Page generated: Tue Aug 6 06:57:06 2024
ISSN: ESSN 1476-4687
PubMed: 28902838
DOI: 10.1038/NATURE23903
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO