Iron »
PDB 5okd-5sx2 »
5omj »
Iron in PDB 5omj: R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion
Enzymatic activity of R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion
All present enzymatic activity of R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion:
1.17.4.1;
1.17.4.1;
Protein crystallography data
The structure of R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion, PDB code: 5omj
was solved by
J.J.Griese,
M.Hogbom,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.66 / 2.01 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 55.625, 97.312, 127.984, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.2 / 26.4 |
Iron Binding Sites:
The binding sites of Iron atom in the R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion
(pdb code 5omj). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion, PDB code: 5omj:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion, PDB code: 5omj:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 5omj
Go back to
Iron binding site 1 out
of 2 in the R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion
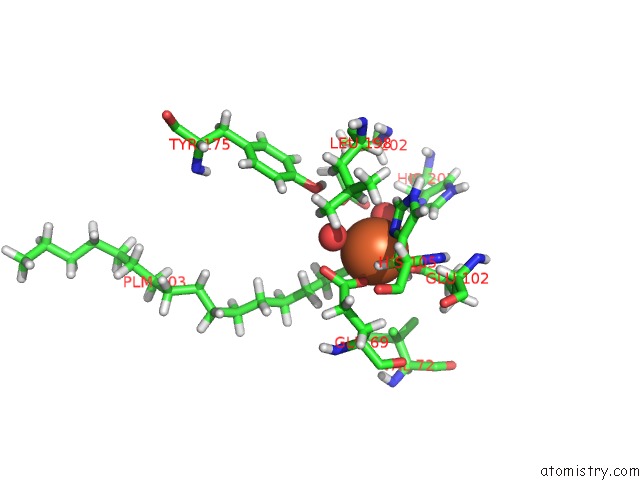
Mono view
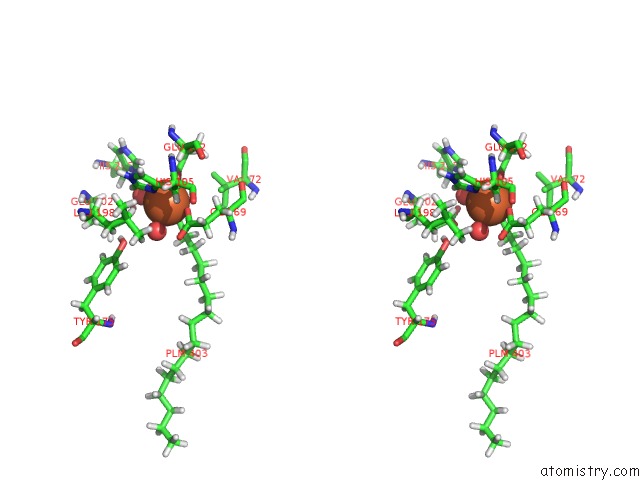
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion within 5.0Å range:
|
Iron binding site 2 out of 2 in 5omj
Go back to
Iron binding site 2 out
of 2 in the R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion

Mono view
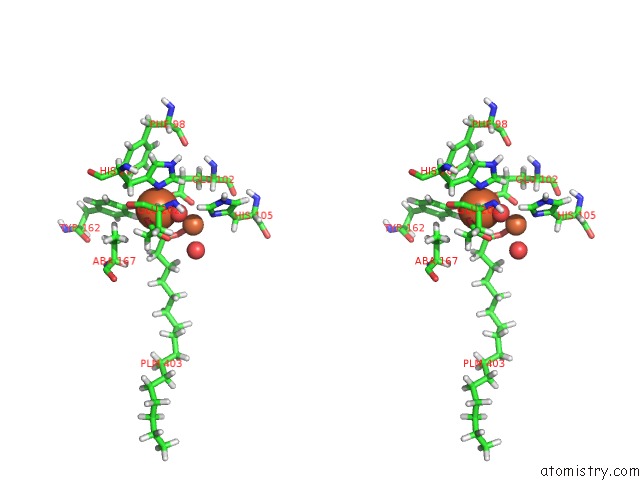
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of R2-Like Ligand-Binding Oxidase with Aerobically Reconstituted Metal Cofactor After Photoconversion within 5.0Å range:
|
Reference:
P.T.Maugeri,
J.J.Griese,
R.M.Branca,
E.K.Miller,
Z.R.Smith,
J.Eirich,
M.Hogbom,
H.S.Shafaat.
Driving Protein Conformational Changes with Light: Photoinduced Structural Rearrangement in A Heterobimetallic Oxidase. J. Am. Chem. Soc. V. 140 1471 2018.
ISSN: ESSN 1520-5126
PubMed: 29268610
DOI: 10.1021/JACS.7B11966
Page generated: Tue Aug 6 08:26:45 2024
ISSN: ESSN 1520-5126
PubMed: 29268610
DOI: 10.1021/JACS.7B11966
Last articles
F in 4JTQF in 4JSC
F in 4JSJ
F in 4JSM
F in 4JQG
F in 4JSI
F in 4JQ2
F in 4JP4
F in 4JPS
F in 4JNC