Iron »
PDB 6g71-6gl6 »
6g94 »
Iron in PDB 6g94: Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
Enzymatic activity of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
All present enzymatic activity of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B:
1.12.99.6;
1.12.99.6;
Protein crystallography data
The structure of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B, PDB code: 6g94
was solved by
A.Volbeda,
J.C.Fontecilla-Camps,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.00 / 2.50 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 124.410, 165.030, 206.220, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.2 / 25.3 |
Other elements in 6g94:
The structure of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B also contains other interesting chemical elements:
| Nickel | (Ni) | 4 atoms |
| Magnesium | (Mg) | 4 atoms |
| Chlorine | (Cl) | 4 atoms |
Iron Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 30; Page 4, Binding sites: 31 - 40; Page 5, Binding sites: 41 - 50;Binding sites:
The binding sites of Iron atom in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B (pdb code 6g94). This binding sites where shown within 5.0 Angstroms radius around Iron atom.In total 50 binding sites of Iron where determined in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B, PDB code: 6g94:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Iron binding site 1 out of 50 in 6g94
Go back to
Iron binding site 1 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
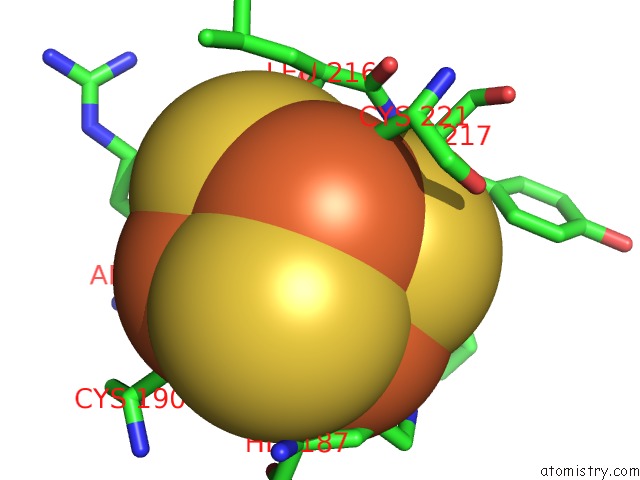
Mono view
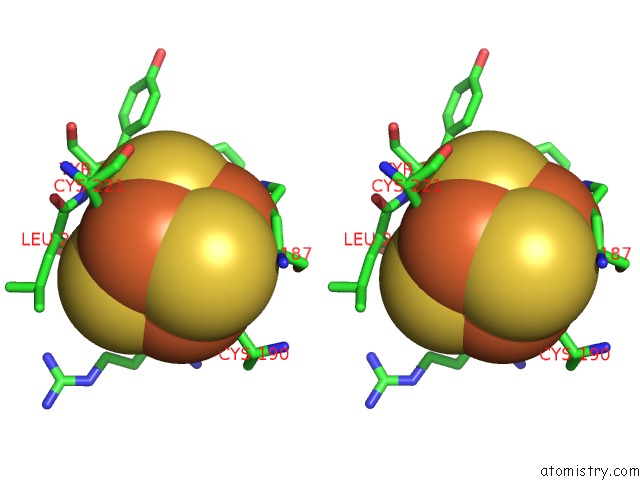
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 2 out of 50 in 6g94
Go back to
Iron binding site 2 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
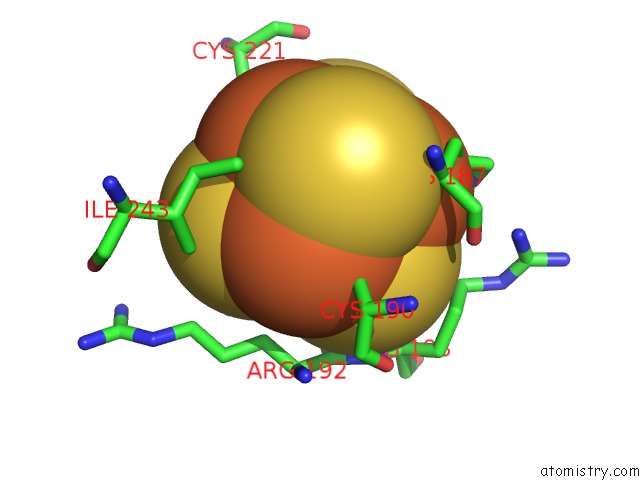
Mono view
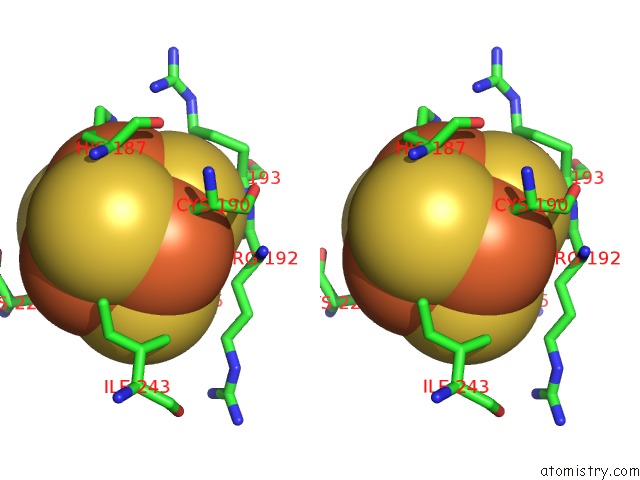
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 3 out of 50 in 6g94
Go back to
Iron binding site 3 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
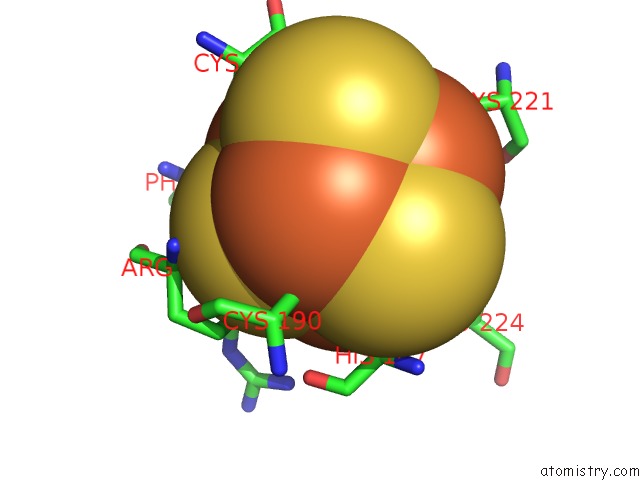
Mono view
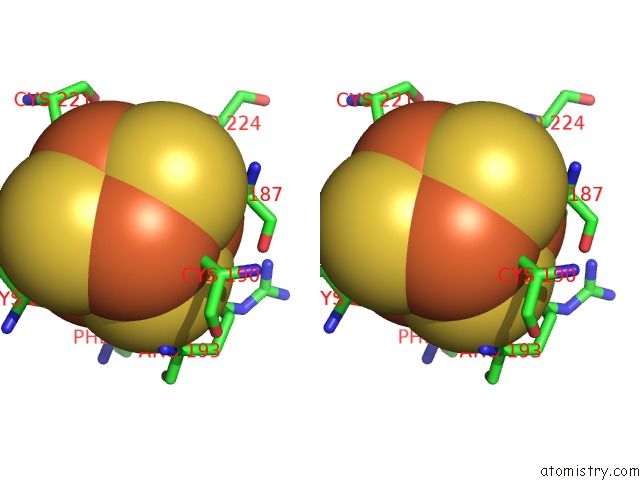
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 4 out of 50 in 6g94
Go back to
Iron binding site 4 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
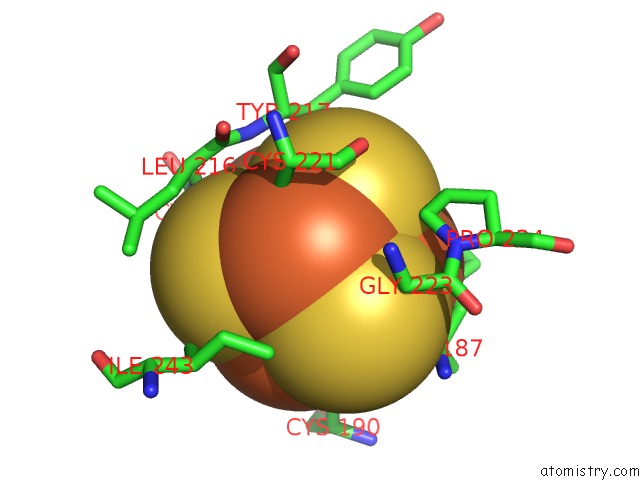
Mono view
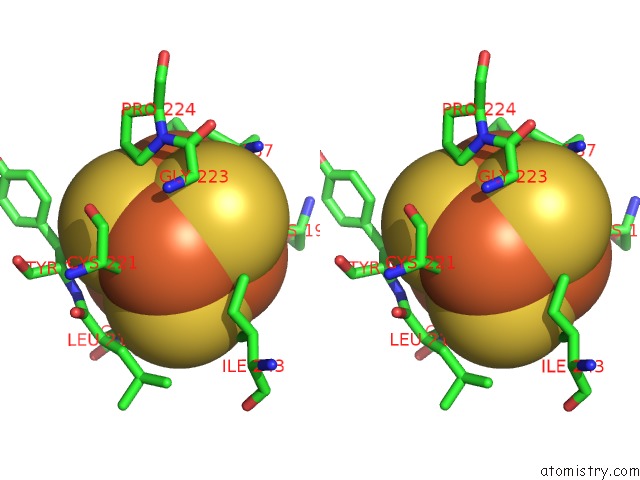
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 5 out of 50 in 6g94
Go back to
Iron binding site 5 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
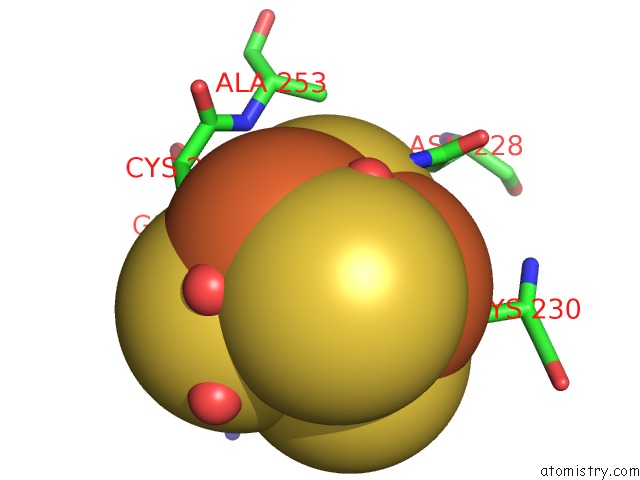
Mono view
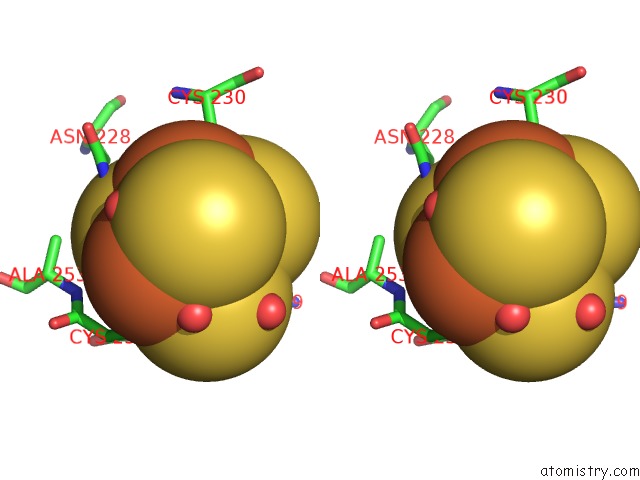
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 6 out of 50 in 6g94
Go back to
Iron binding site 6 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
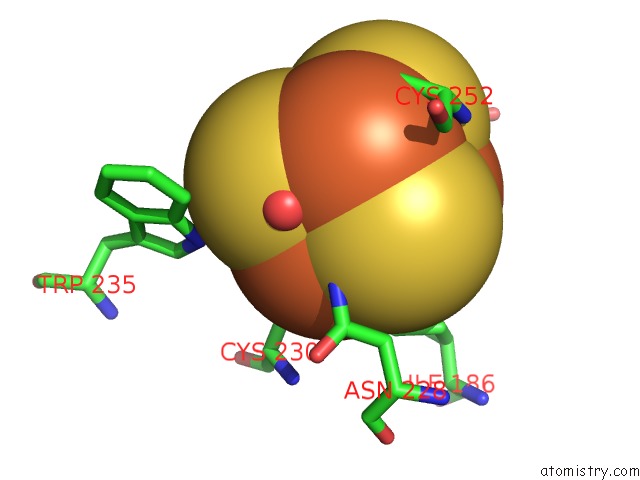
Mono view
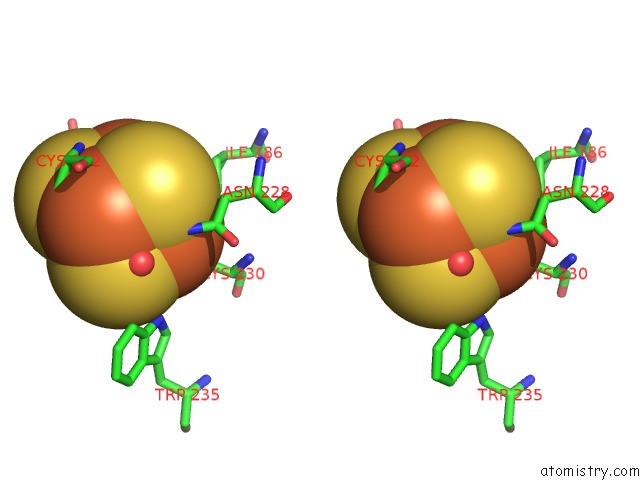
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 7 out of 50 in 6g94
Go back to
Iron binding site 7 out
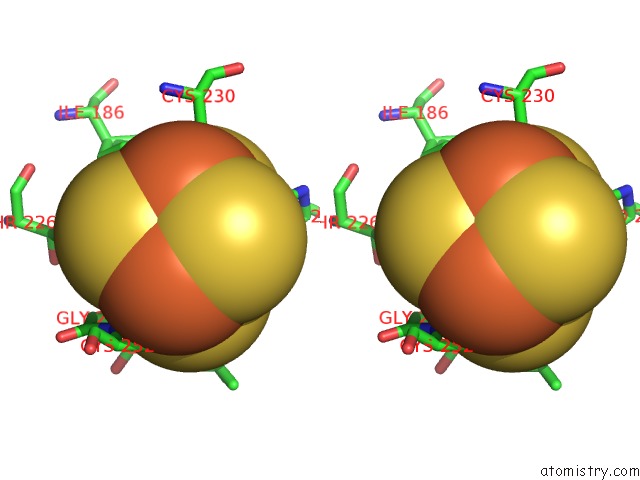
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
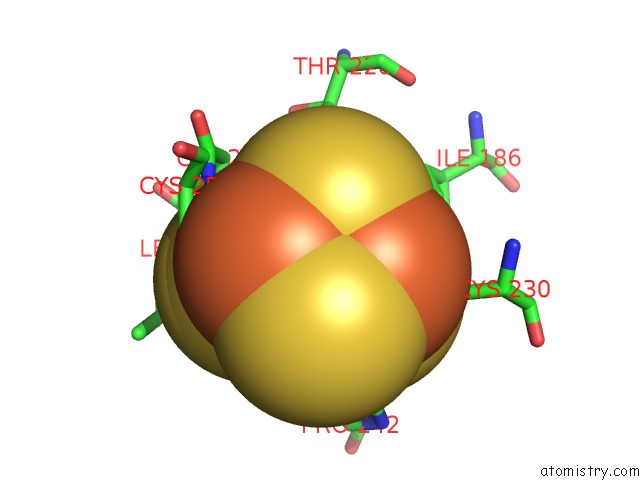
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 7 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 8 out of 50 in 6g94
Go back to
Iron binding site 8 out
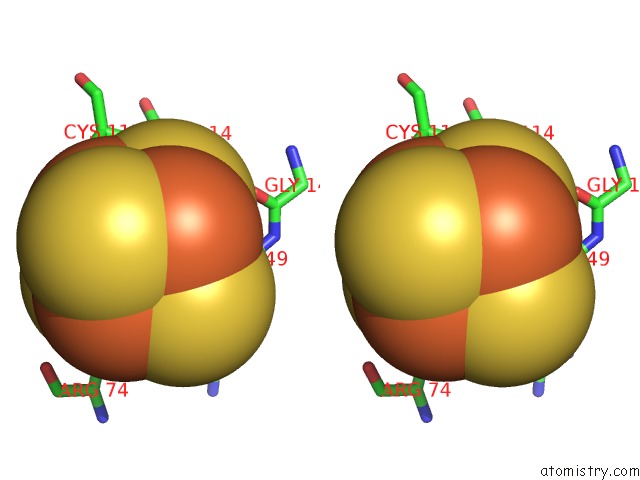
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
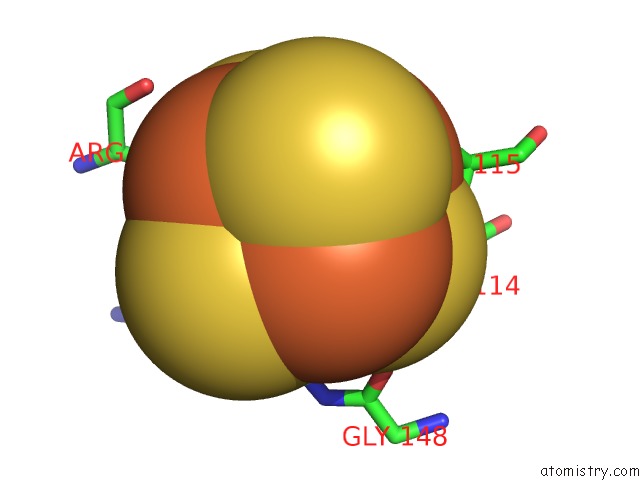
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 8 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 9 out of 50 in 6g94
Go back to
Iron binding site 9 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
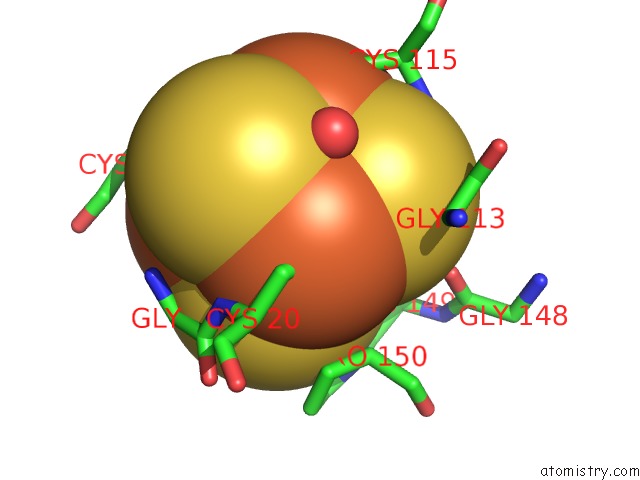
Mono view
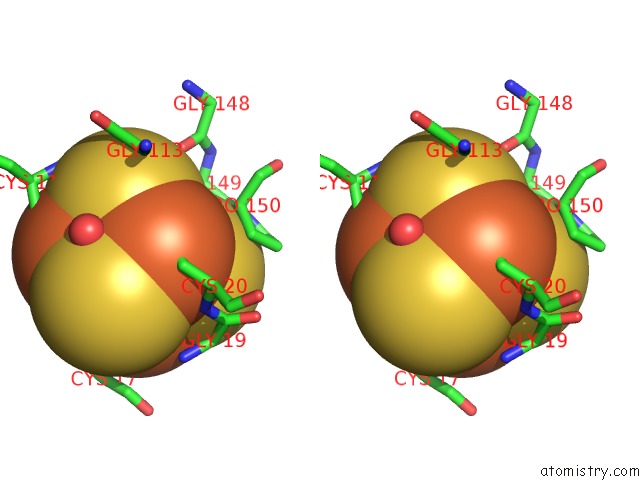
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 9 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Iron binding site 10 out of 50 in 6g94
Go back to
Iron binding site 10 out
of 50 in the Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B
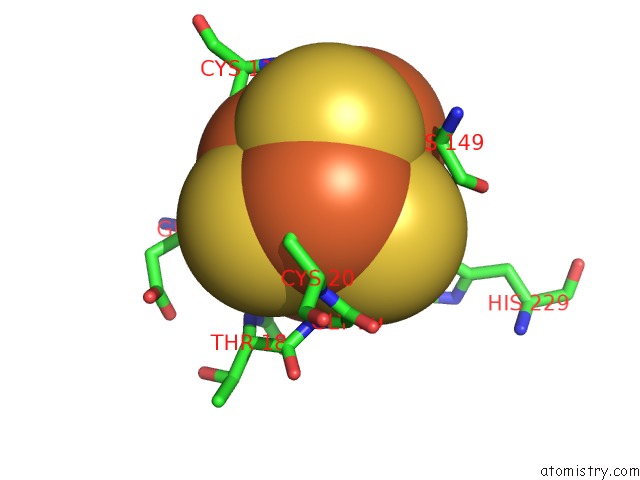
Mono view
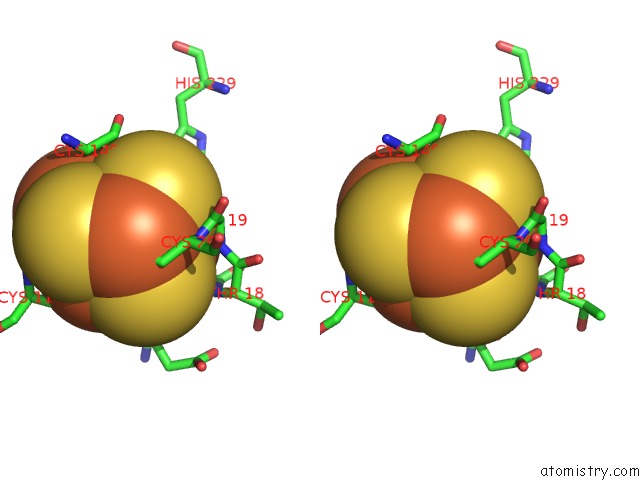
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 10 of Structure of E. Coli Hydrogenase-1 C19G Variant in Complex with Cytochrome B within 5.0Å range:
|
Reference:
A.Volbeda,
J.M.Mouesca,
C.Darnault,
M.M.Roessler,
A.Parkin,
F.A.Armstrong,
J.C.Fontecilla-Camps.
X-Ray Structural, Functional and Computational Studies of the O2-Sensitive E. Coli Hydrogenase-1 C19G Variant Reveal An Unusual [4FE-4S] Cluster. Chem. Commun. (Camb.) V. 54 7175 2018.
ISSN: ESSN 1364-548X
PubMed: 29888350
DOI: 10.1039/C8CC02896F
Page generated: Tue Aug 6 19:12:02 2024
ISSN: ESSN 1364-548X
PubMed: 29888350
DOI: 10.1039/C8CC02896F
Last articles
Cl in 8CHYCl in 8CHR
Cl in 8CHQ
Cl in 8CHP
Cl in 8CHL
Cl in 8CHJ
Cl in 8CHN
Cl in 8CHK
Cl in 8CHI
Cl in 8CGS