Iron »
PDB 6pq6-6qgr »
6q6q »
Iron in PDB 6q6q: Human Aldehyde Oxidase Snp G1269R
Enzymatic activity of Human Aldehyde Oxidase Snp G1269R
All present enzymatic activity of Human Aldehyde Oxidase Snp G1269R:
1.2.3.1;
1.2.3.1;
Protein crystallography data
The structure of Human Aldehyde Oxidase Snp G1269R, PDB code: 6q6q
was solved by
C.Mota,
C.Coelho,
T.Santos-Silva,
M.J.Romao,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.86 / 3.10 |
| Space group | P 42 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 148.197, 148.197, 132.204, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.2 / 23.6 |
Iron Binding Sites:
The binding sites of Iron atom in the Human Aldehyde Oxidase Snp G1269R
(pdb code 6q6q). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 4 binding sites of Iron where determined in the Human Aldehyde Oxidase Snp G1269R, PDB code: 6q6q:
Jump to Iron binding site number: 1; 2; 3; 4;
In total 4 binding sites of Iron where determined in the Human Aldehyde Oxidase Snp G1269R, PDB code: 6q6q:
Jump to Iron binding site number: 1; 2; 3; 4;
Iron binding site 1 out of 4 in 6q6q
Go back to
Iron binding site 1 out
of 4 in the Human Aldehyde Oxidase Snp G1269R
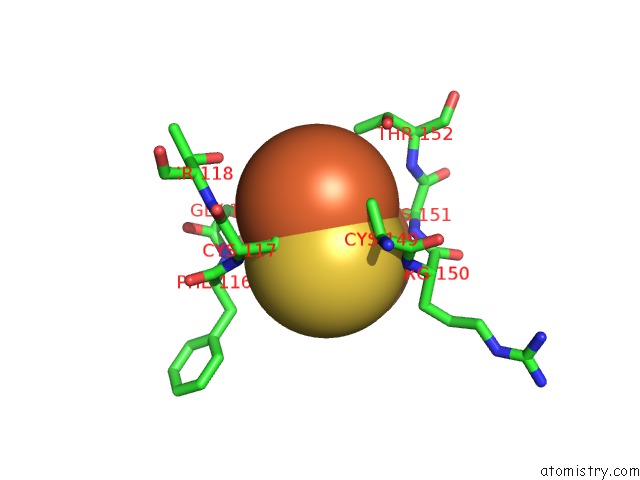
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Human Aldehyde Oxidase Snp G1269R within 5.0Å range:
|
Iron binding site 2 out of 4 in 6q6q
Go back to
Iron binding site 2 out
of 4 in the Human Aldehyde Oxidase Snp G1269R

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Human Aldehyde Oxidase Snp G1269R within 5.0Å range:
|
Iron binding site 3 out of 4 in 6q6q
Go back to
Iron binding site 3 out
of 4 in the Human Aldehyde Oxidase Snp G1269R

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Human Aldehyde Oxidase Snp G1269R within 5.0Å range:
|
Iron binding site 4 out of 4 in 6q6q
Go back to
Iron binding site 4 out
of 4 in the Human Aldehyde Oxidase Snp G1269R

Mono view
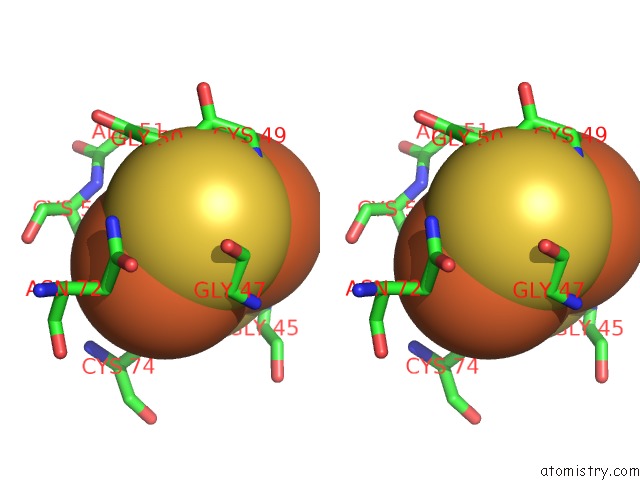
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Human Aldehyde Oxidase Snp G1269R within 5.0Å range:
|
Reference:
C.Mota,
M.Esmaeeli,
C.Coelho,
T.Santos-Silva,
M.Wolff,
A.Foti,
S.Leimkuhler,
M.J.Romao.
Human Aldehyde Oxidase (HAOX1): Structure Determination of the Moco-Free Form of the Natural Variant G1269R and Biophysical Studies of Single Nucleotide Polymorphisms. Febs Open Bio V. 9 925 2019.
ISSN: ESSN 2211-5463
PubMed: 30985987
DOI: 10.1002/2211-5463.12617
Page generated: Wed Aug 6 12:08:48 2025
ISSN: ESSN 2211-5463
PubMed: 30985987
DOI: 10.1002/2211-5463.12617
Last articles
Fe in 6UO0Fe in 6UOI
Fe in 6UNM
Fe in 6UNN
Fe in 6UNL
Fe in 6UNJ
Fe in 6UNC
Fe in 6UNK
Fe in 6UNI
Fe in 6UNH