Iron »
PDB 6qgr-6r2o »
6qq5 »
Iron in PDB 6qq5: Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
Enzymatic activity of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
All present enzymatic activity of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans:
1.7.2.5;
1.7.2.5;
Other elements in 6qq5:
The structure of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans also contains other interesting chemical elements:
| Calcium | (Ca) | 2 atoms |
Iron Binding Sites:
The binding sites of Iron atom in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
(pdb code 6qq5). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 6 binding sites of Iron where determined in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans, PDB code: 6qq5:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Iron where determined in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans, PDB code: 6qq5:
Jump to Iron binding site number: 1; 2; 3; 4; 5; 6;
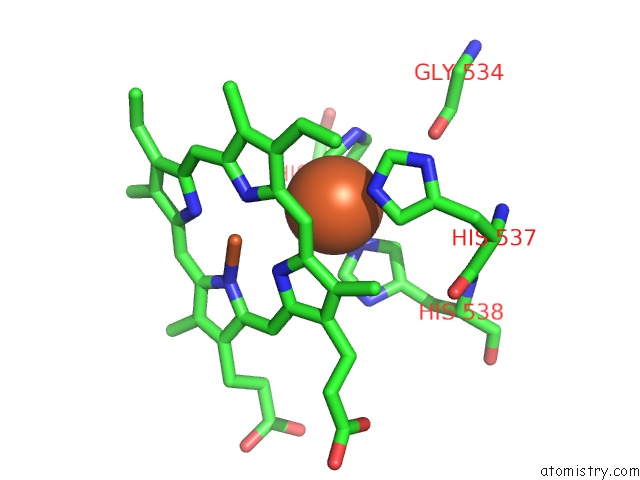
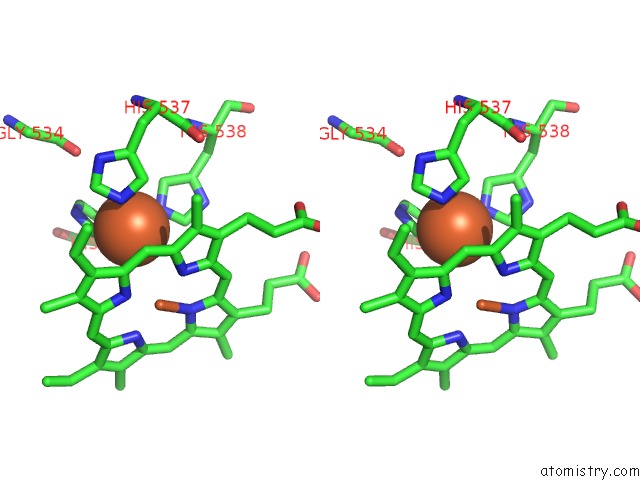
Iron binding site 1 out of 6 in 6qq5
Go back to
Iron binding site 1 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
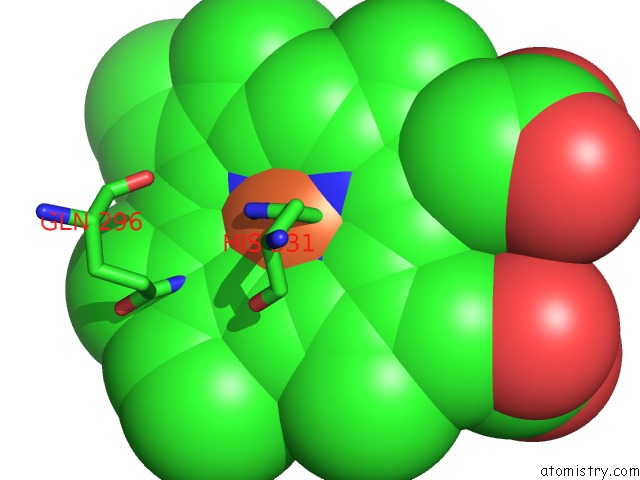
Mono view
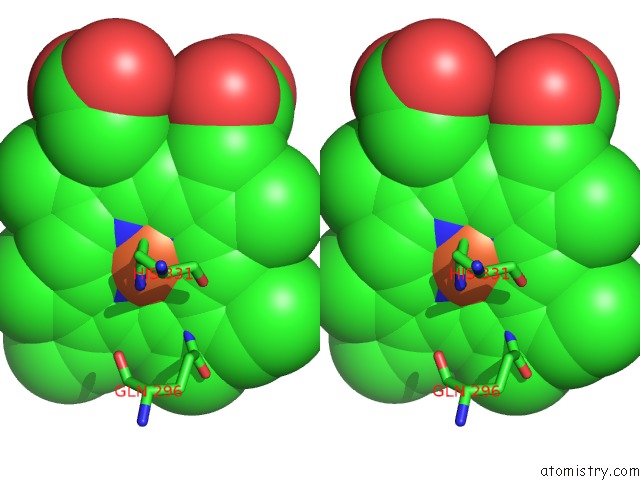
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
Iron binding site 2 out of 6 in 6qq5
Go back to
Iron binding site 2 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
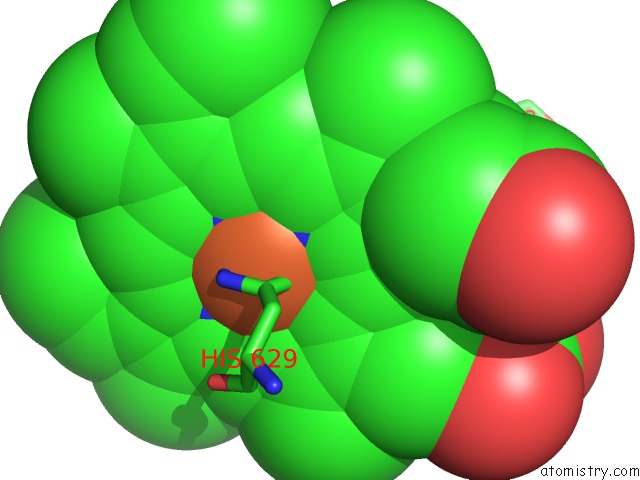
Mono view
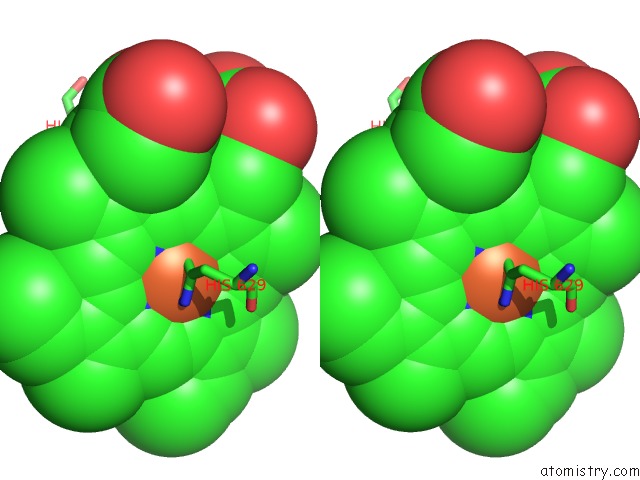
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
Iron binding site 3 out of 6 in 6qq5
Go back to
Iron binding site 3 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 3 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
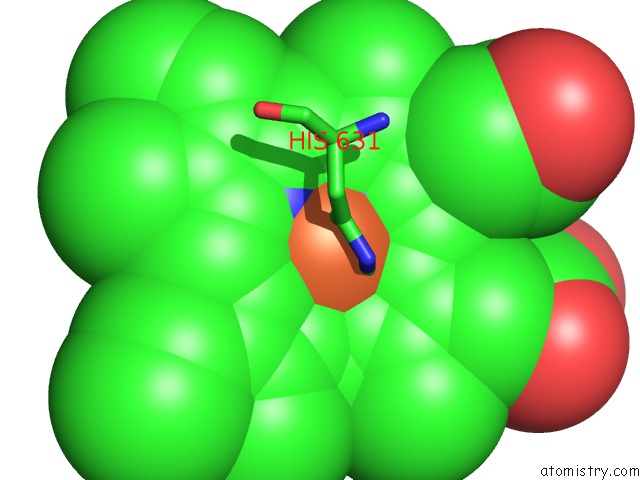
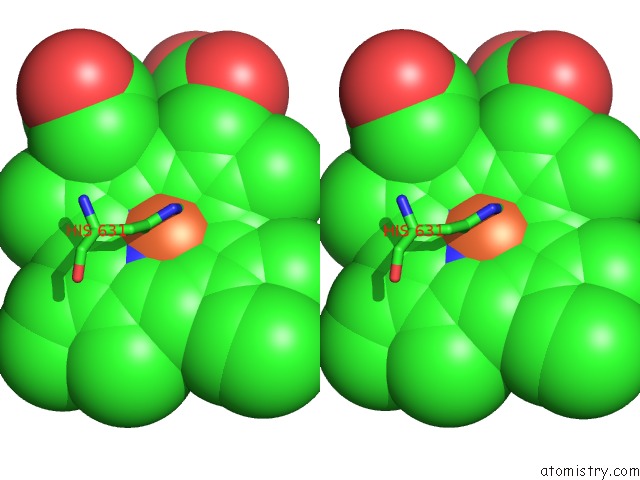
Iron binding site 4 out of 6 in 6qq5
Go back to
Iron binding site 4 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 4 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
Iron binding site 5 out of 6 in 6qq5
Go back to
Iron binding site 5 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
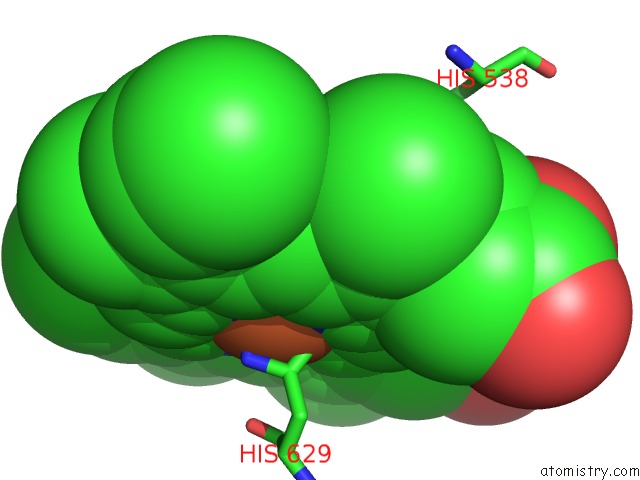
Mono view
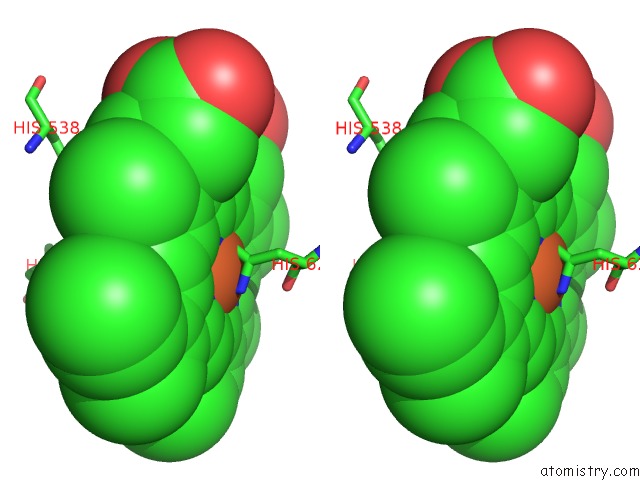
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 5 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
Iron binding site 6 out of 6 in 6qq5
Go back to
Iron binding site 6 out
of 6 in the Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans
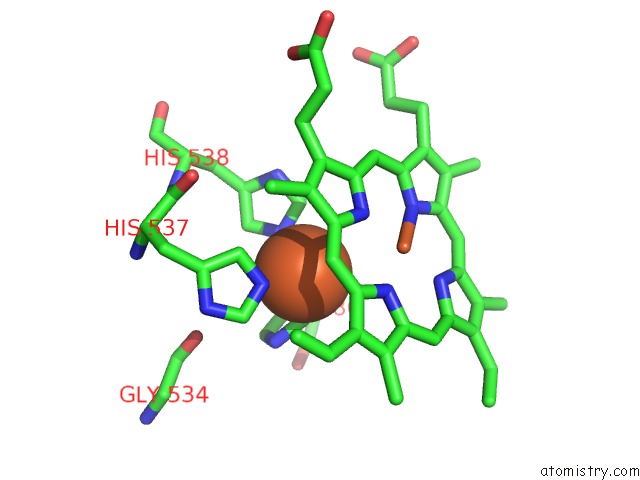
Mono view
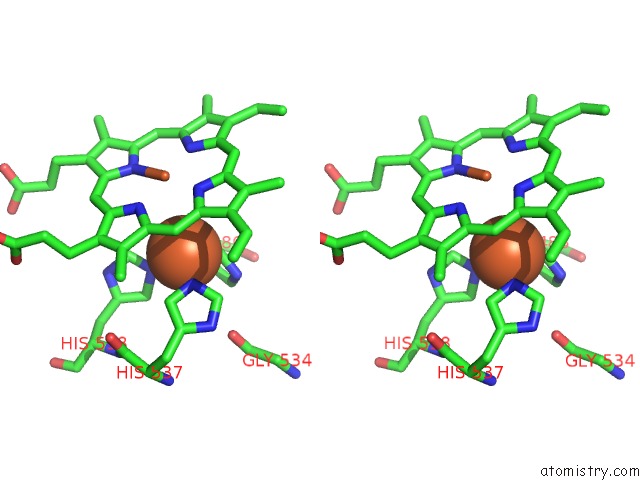
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 6 of Cryo-Em Structure of Dimeric Quinol Dependent Nitric Oxide Reductase (Qnor) From Alcaligenes Xylosoxidans within 5.0Å range:
|
Reference:
C.C.Gopalasingam,
R.M.Johnson,
G.N.Chiduza,
T.Tosha,
M.Yamamoto,
Y.Shiro,
S.V.Antonyuk,
S.P.Muench,
S.S.Hasnain.
Dimeric Structures of Quinol-Dependent Nitric Oxide Reductases (Qnors) Revealed By Cryo-Electron Microscopy. Sci Adv V. 5 X1803 2019.
ISSN: ESSN 2375-2548
PubMed: 31489376
DOI: 10.1126/SCIADV.AAX1803
Page generated: Wed Aug 7 08:17:33 2024
ISSN: ESSN 2375-2548
PubMed: 31489376
DOI: 10.1126/SCIADV.AAX1803
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF