Iron »
PDB 6r3w-6rr4 »
6r5s »
Iron in PDB 6r5s: Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II)
Protein crystallography data
The structure of Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II), PDB code: 6r5s
was solved by
S.Morera,
A.Vigouroux,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 37.62 / 2.75 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 72.010, 44.190, 179.760, 90.00, 95.06, 90.00 |
| R / Rfree (%) | 18.9 / 24.2 |
Iron Binding Sites:
The binding sites of Iron atom in the Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II)
(pdb code 6r5s). This binding sites where shown within
5.0 Angstroms radius around Iron atom.
In total 2 binding sites of Iron where determined in the Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II), PDB code: 6r5s:
Jump to Iron binding site number: 1; 2;
In total 2 binding sites of Iron where determined in the Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II), PDB code: 6r5s:
Jump to Iron binding site number: 1; 2;
Iron binding site 1 out of 2 in 6r5s
Go back to
Iron binding site 1 out
of 2 in the Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II)
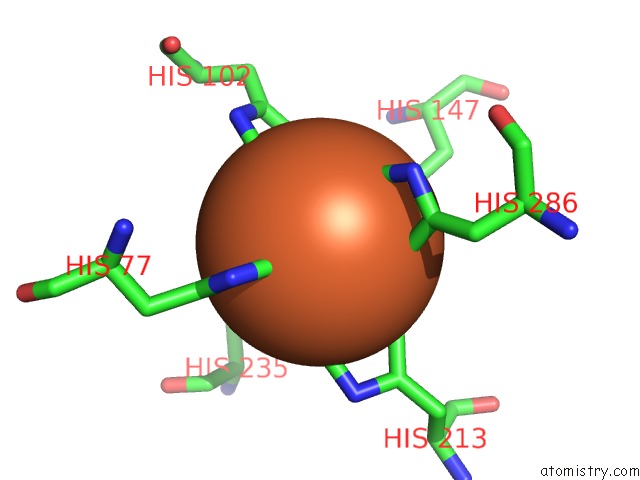
Mono view
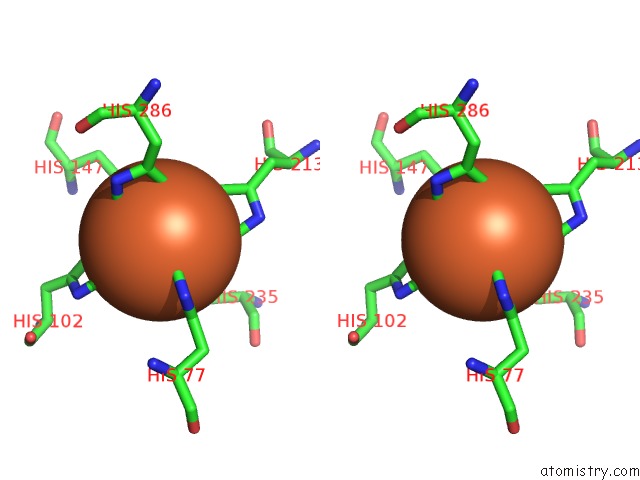
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 1 of Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II) within 5.0Å range:
|
Iron binding site 2 out of 2 in 6r5s
Go back to
Iron binding site 2 out
of 2 in the Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II)
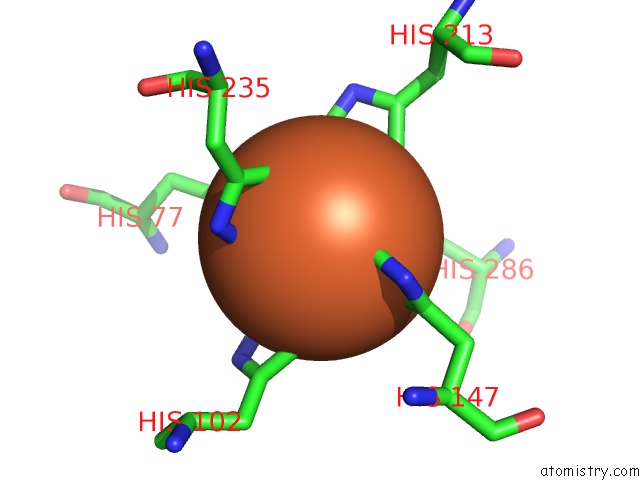
Mono view
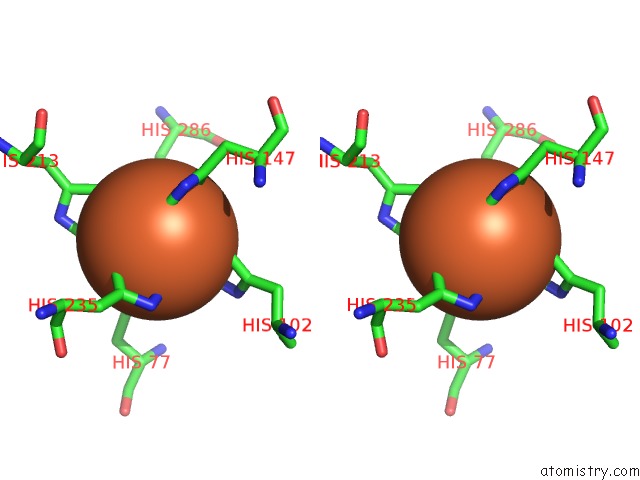
Stereo pair view

Mono view

Stereo pair view
A full contact list of Iron with other atoms in the Fe binding
site number 2 of Structure of the Sbp Fpvc From Pseudomonas Aeruginosa in Complex with Fe(II) within 5.0Å range:
|
Reference:
A.Vigouroux,
M.Aumont-Nicaise,
A.Boussac,
L.Marty,
L.Lo Bello,
P.Legrand,
K.Brillet,
I.J.Schalk,
S.Morera.
A Unique Ferrous Iron Binding Mode Is Associated with Large Conformational Changes For the Transport Protein Fpvc of Pseudomonas Aeruginosa. Febs J. 2019.
ISSN: ISSN 1742-464X
PubMed: 31318478
DOI: 10.1111/FEBS.15004
Page generated: Wed Aug 6 12:54:33 2025
ISSN: ISSN 1742-464X
PubMed: 31318478
DOI: 10.1111/FEBS.15004
Last articles
Fe in 6VSHFe in 6VRI
Fe in 6VP5
Fe in 6VLT
Fe in 6VP4
Fe in 6VP3
Fe in 6VP1
Fe in 6VP2
Fe in 6VO9
Fe in 6VOZ